The Presence of Human Herpesvirus 6 in the Brain in Health and Disease
- PMID: 33172107
- PMCID: PMC7694807
- DOI: 10.3390/biom10111520
The Presence of Human Herpesvirus 6 in the Brain in Health and Disease
Abstract
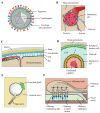
The human herpesvirus 6 (HHV-6) -A and -B are two dsDNA beta-herpesviruses infectingalmost the entire worldwide population. These viruses have been implicated in multipleneurological conditions in individuals of various ages and immunological status, includingencephalitis, epilepsy, and febrile seizures. HHV-6s have also been suggested as playing a role inthe etiology of neurodegenerative diseases such as multiple sclerosis and Alzheimer's disease. Theapparent robustness of these suggested associations is contingent on the accuracy of HHV-6detection in the nervous system. The effort of more than three decades of researching HHV-6 in thebrain has yielded numerous observations, albeit using variable technical approaches in terms oftissue preservation, detection techniques, sample sizes, brain regions, and comorbidities. In thisreview, we aimed to summarize current knowledge about the entry routes and direct presence ofHHV-6 in the brain parenchyma at the level of DNA, RNA, proteins, and specific cell types, inhealthy subjects and in those with neurological conditions. We also discuss recent findings relatedto the presence of HHV-6 in the brains of patients with Alzheimer's disease in light of availableevidence.
Keywords: Alzheimer’s disease; brain; encephalitis; epilepsy; human herpesvirus 6; immunohistochemistry; multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Multiscale Analysis of Independent Alzheimer's Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus.Neuron. 2018 Jul 11;99(1):64-82.e7. doi: 10.1016/j.neuron.2018.05.023. Epub 2018 Jun 21. Neuron. 2018. PMID: 29937276 Free PMC article.
-
A Murine Herpesvirus Closely Related to Ubiquitous Human Herpesviruses Causes T-Cell Depletion.J Virol. 2017 Apr 13;91(9):e02463-16. doi: 10.1128/JVI.02463-16. Print 2017 May 1. J Virol. 2017. PMID: 28179532 Free PMC article.
-
Association of human herpesvirus-6B with mesial temporal lobe epilepsy.PLoS Med. 2007 May;4(5):e180. doi: 10.1371/journal.pmed.0040180. PLoS Med. 2007. PMID: 17535102 Free PMC article.
-
Human Herpesviruses 6A and 6B in Brain Diseases: Association versus Causation.Clin Microbiol Rev. 2020 Nov 11;34(1):e00143-20. doi: 10.1128/CMR.00143-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33177186 Free PMC article. Review.
-
Infection with HHV-6 and its role in epilepsy.Epilepsy Res. 2019 Jul;153:34-39. doi: 10.1016/j.eplepsyres.2019.03.016. Epub 2019 Mar 29. Epilepsy Res. 2019. PMID: 30953871 Review.
Cited by
-
Symptomatic central nervous system tuberculosis and human herpesvirus-6 coinfection with associated hydrocephalus managed with endoscopic third ventriculostomy: A case report and review of human herpesvirus-6 neuropathology.Surg Neurol Int. 2024 Aug 16;15:287. doi: 10.25259/SNI_355_2024. eCollection 2024. Surg Neurol Int. 2024. PMID: 39246759 Free PMC article.
-
Humans with inherited MyD88 and IRAK-4 deficiencies are predisposed to hypoxemic COVID-19 pneumonia.J Exp Med. 2023 May 1;220(5):e20220170. doi: 10.1084/jem.20220170. Epub 2023 Mar 3. J Exp Med. 2023. PMID: 36880831 Free PMC article.
-
TRIM Proteins: Key Regulators of Immunity to Herpesvirus Infection.Viruses. 2024 Nov 6;16(11):1738. doi: 10.3390/v16111738. Viruses. 2024. PMID: 39599852 Free PMC article. Review.
-
Viruses and the Brain-A Relationship Prone to Trouble.Viruses. 2025 Jan 31;17(2):203. doi: 10.3390/v17020203. Viruses. 2025. PMID: 40006958 Free PMC article. Review.
-
Differential Impacts of HHV-6A versus HHV-6B Infection in Differentiated Human Neural Stem Cells.Front Immunol. 2022 Jul 13;13:847106. doi: 10.3389/fimmu.2022.847106. eCollection 2022. Front Immunol. 2022. PMID: 35911725 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

