Population Pharmacokinetic Analysis of Amikacin for Optimal Pharmacotherapy in Korean Patients with Nontuberculous Mycobacterial Pulmonary Disease
- PMID: 33172135
- PMCID: PMC7694782
- DOI: 10.3390/antibiotics9110784
Population Pharmacokinetic Analysis of Amikacin for Optimal Pharmacotherapy in Korean Patients with Nontuberculous Mycobacterial Pulmonary Disease
Abstract
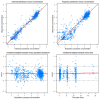
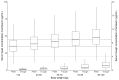
Amikacin is used as a therapy for patients with nontuberculous mycobacterial pulmonary disease (NTM-PD) who are resistant to macrolide antibiotics or have severe symptoms. This study aimed to characterize the pharmacokinetic properties of amikacin in patients with NTM-PD by developing a population pharmacokinetic model and to explore the optimal pharmacotherapy in patients with NTM-PD. For this study, all data were retrospectively collected. The amikacin pharmacokinetic properties were best described by a two-compartment model with first-order elimination. The estimated glomerular filtration rate and body weight were identified as significant covariates for clearance and the volume of distribution, respectively. A model-based simulation was conducted to explore the probability of reaching the target therapeutic range when various dose regimens were administered according to the body weight and renal function. The simulation results indicated that the amikacin dosage should be determined based on the body weight, and for patients who weigh over 70 kg, it is necessary to adjust the dose according to renal function. In conclusion, the optimal pharmacotherapy of amikacin for patients with NTM-PD was recommended based on the population pharmacokinetic model, which is expected to enable the personalization of drug therapy and improve the clinical outcomes of amikacin therapy.
Keywords: amikacin; nontuberculous mycobacterial pulmonary disease; population pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Failure to predict amikacin elimination in critically ill patients with cancer based on the estimated glomerular filtration rate: applying PBPK approach in a therapeutic drug monitoring study.Eur J Clin Pharmacol. 2023 Jul;79(7):1003-1012. doi: 10.1007/s00228-023-03516-1. Epub 2023 May 31. Eur J Clin Pharmacol. 2023. PMID: 37256410
-
Safety and effectiveness of low-dose amikacin in nontuberculous mycobacterial pulmonary disease treated in Toronto, Canada.BMC Pharmacol Toxicol. 2019 Jun 3;20(1):37. doi: 10.1186/s40360-019-0302-1. BMC Pharmacol Toxicol. 2019. PMID: 31159865 Free PMC article.
-
Amikacin Inhalation as Salvage Therapy for Refractory Nontuberculous Mycobacterial Lung Disease.Antimicrob Agents Chemother. 2018 Jun 26;62(7):e00011-18. doi: 10.1128/AAC.00011-18. Print 2018 Jul. Antimicrob Agents Chemother. 2018. PMID: 29661870 Free PMC article.
-
Interrelational changes in the epidemiology and clinical features of nontuberculous mycobacterial pulmonary disease and tuberculosis in a referral hospital in Japan.Respir Med. 2019 Jun;152:74-80. doi: 10.1016/j.rmed.2019.05.001. Epub 2019 May 8. Respir Med. 2019. PMID: 31128614 Review.
-
Clinical Pharmacokinetics of Amikacin in Pediatric Patients: A Comprehensive Review of Population Pharmacokinetic Analyses.Clin Pharmacokinet. 2018 Oct;57(10):1217-1228. doi: 10.1007/s40262-018-0641-x. Clin Pharmacokinet. 2018. PMID: 29572662 Review.
Cited by
-
Population Pharmacokinetics of Difloxacin in Crucian Carp (Carassius auratus) after a Single Oral Administration.Vet Sci. 2023 Jun 27;10(7):416. doi: 10.3390/vetsci10070416. Vet Sci. 2023. PMID: 37505822 Free PMC article.
-
Nitric oxide-releasing prodrug for the treatment of complex Mycobacterium abscessus infections.Antimicrob Agents Chemother. 2024 Feb 7;68(2):e0132723. doi: 10.1128/aac.01327-23. Epub 2024 Jan 11. Antimicrob Agents Chemother. 2024. PMID: 38206003 Free PMC article.
-
The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023.Microorganisms. 2024 Aug 8;12(8):1615. doi: 10.3390/microorganisms12081615. Microorganisms. 2024. PMID: 39203457 Free PMC article.
-
A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases.J Nanobiotechnology. 2022 Mar 3;20(1):101. doi: 10.1186/s12951-022-01307-x. J Nanobiotechnology. 2022. PMID: 35241085 Free PMC article. Review.
References
-
- Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., Holland S.M., Horsburgh R., Huitt G., Iademarco M.F., et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Resp. Crit. Care. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. - DOI - PubMed
-
- Burton M.E. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2006.
LinkOut - more resources
Full Text Sources

