Study of the Photothermal Catalytic Mechanism of CO2 Reduction to CH4 by Ruthenium Nanoparticles Supported on Titanate Nanotubes
- PMID: 33172154
- PMCID: PMC7694752
- DOI: 10.3390/nano10112212
Study of the Photothermal Catalytic Mechanism of CO2 Reduction to CH4 by Ruthenium Nanoparticles Supported on Titanate Nanotubes
Abstract
The Sabatier reaction could be a key tool for the future of the renewable energy field due to the potential of this reaction to produce either fuels or to stabilize H2 in the form of stable chemicals. For this purpose, a new composite made of ruthenium oxide nanoparticles (NPs) deposited on titanate nanotubes (TiNTs) was tested. Titanate nanotubes are a robust semiconductor with a one-dimensional (1D) morphology that results in a high contact area making this material suitable for photocatalysis. Small ruthenium nanoparticles (1.5 nm) were deposited on TiNTs at different ratios by Na+-to-Ru3+ ion exchanges followed by calcination. These samples were tested varying light power and temperature conditions to study the reaction mechanism during catalysis. Methanation of CO2 catalyzed by Ru/TiNT composite exhibit photonic and thermic contributions, and their ratios vary with temperature and light intensity. The synthesized composite achieved a production rate of 12.4 mmol CH4·gcat-1·h-1 equivalent to 110.7 mmol of CH4·gRu-1·h-1 under 150 mW/cm2 simulated sunlight irradiation at 210 °C. It was found that photo-response derives either from Ru nanoparticle excitation in the visible (VIS) and near-infrared (NIR) region (photothermal and plasmon excitation mechanism) or from TiNT excitation in the ultraviolet (UV) region leading to electron-hole separation and photoinduced electron transfer.
Keywords: CO2 reduction; Sabatier reaction; methanation; methane; photocatalysis; photothermal catalysis; ruthenium nanoparticles; solar fuels; titanate nanotubes; titanates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

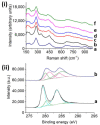


 ).
).



Similar articles
-
Synergism of Au and Ru Nanoparticles in Low-Temperature Photoassisted CO2 Methanation.Chemistry. 2018 Dec 10;24(69):18436-18443. doi: 10.1002/chem.201803022. Epub 2018 Oct 12. Chemistry. 2018. PMID: 30125410
-
Reinforcing the Efficiency of Photothermal Catalytic CO2 Methanation through Integration of Ru Nanoparticles with Photothermal MnCo2O4 Nanosheets.ACS Nano. 2023 Dec 12;17(23):23761-23771. doi: 10.1021/acsnano.3c07630. Epub 2023 Nov 20. ACS Nano. 2023. PMID: 37982387
-
Enhanced Visible Light-Driven Photocatalytic Water-Splitting Reaction of Titanate Nanotubes Sensitised with Ru(II) Bipyridyl Complex.Nanomaterials (Basel). 2023 Nov 16;13(22):2959. doi: 10.3390/nano13222959. Nanomaterials (Basel). 2023. PMID: 37999313 Free PMC article.
-
Accelerating photo-thermal CO2 reduction to CO, CH4 or methanol over metal/oxide semiconductor catalysts.iScience. 2022 Mar 18;25(4):104107. doi: 10.1016/j.isci.2022.104107. eCollection 2022 Apr 15. iScience. 2022. PMID: 35378856 Free PMC article. Review.
-
Construction of robust Ni-based catalysts for low-temperature Sabatier reaction.Chem Commun (Camb). 2024 Oct 8;60(81):11466-11482. doi: 10.1039/d4cc04342a. Chem Commun (Camb). 2024. PMID: 39279413 Review.
Cited by
-
Photocatalytic Water Splitting Promoted by 2D and 3D Porphyrin Covalent Organic Polymers Synthesized by Suzuki-Miyaura Carbon-Carbon Coupling.Nanomaterials (Basel). 2022 Sep 14;12(18):3197. doi: 10.3390/nano12183197. Nanomaterials (Basel). 2022. PMID: 36144987 Free PMC article.
-
Understanding the Light-Driven Enhancement of CO2 Hydrogenation over Ru/TiO2 Catalysts.Molecules. 2025 Jun 13;30(12):2577. doi: 10.3390/molecules30122577. Molecules. 2025. PMID: 40572541 Free PMC article.
-
Catalytic Methane Decomposition to Carbon Nanostructures and COx-Free Hydrogen: A Mini-Review.Nanomaterials (Basel). 2021 May 6;11(5):1226. doi: 10.3390/nano11051226. Nanomaterials (Basel). 2021. PMID: 34066547 Free PMC article. Review.
-
Photoactive nanomaterials enabled integrated photo-rechargeable batteries.Nanophotonics. 2022 Apr 6;11(8):1443-1484. doi: 10.1515/nanoph-2021-0782. eCollection 2022 Mar. Nanophotonics. 2022. PMID: 39635284 Free PMC article. Review.
References
-
- Lan Y., Gao X.-P., Tana T., Zheng Z.F., Yan T.Y., Wu F., Ringer S.P., Song D.Y. Titanate Nanotubes and Nanorods Prepared from Rutile Powder. Adv. Funct. Mater. 2005;15:1310–1318. doi: 10.1002/adfm.200400353. - DOI
-
- Saeidi S., Amin N.A.S., Rahimpour M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014;5:66–81. doi: 10.1016/j.jcou.2013.12.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

