Thermodynamics and Kinetics of Glycolytic Reactions. Part I: Kinetic Modeling Based on Irreversible Thermodynamics and Validation by Calorimetry
- PMID: 33172189
- PMCID: PMC7664384
- DOI: 10.3390/ijms21218341
Thermodynamics and Kinetics of Glycolytic Reactions. Part I: Kinetic Modeling Based on Irreversible Thermodynamics and Validation by Calorimetry
Abstract
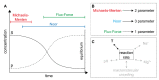
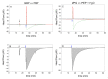
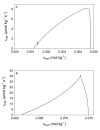
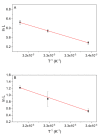
In systems biology, material balances, kinetic models, and thermodynamic boundary conditions are increasingly used for metabolic network analysis. It is remarkable that the reversibility of enzyme-catalyzed reactions and the influence of cytosolic conditions are often neglected in kinetic models. In fact, enzyme-catalyzed reactions in numerous metabolic pathways such as in glycolysis are often reversible, i.e., they only proceed until an equilibrium state is reached and not until the substrate is completely consumed. Here, we propose the use of irreversible thermodynamics to describe the kinetic approximation to the equilibrium state in a consistent way with very few adjustable parameters. Using a flux-force approach allowed describing the influence of cytosolic conditions on the kinetics by only one single parameter. The approach was applied to reaction steps 2 and 9 of glycolysis (i.e., the phosphoglucose isomerase reaction from glucose 6-phosphate to fructose 6-phosphate and the enolase-catalyzed reaction from 2-phosphoglycerate to phosphoenolpyruvate and water). The temperature dependence of the kinetic parameter fulfills the Arrhenius relation and the derived activation energies are plausible. All the data obtained in this work were measured efficiently and accurately by means of isothermal titration calorimetry (ITC). The combination of calorimetric monitoring with simple flux-force relations has the potential for adequate consideration of cytosolic conditions in a simple manner.
Keywords: biothermodynamics; glycolysis; isothermal titration calorimetry; systems biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Thermodynamics and Kinetics of Glycolytic Reactions. Part II: Influence of Cytosolic Conditions on Thermodynamic State Variables and Kinetic Parameters.Int J Mol Sci. 2020 Oct 25;21(21):7921. doi: 10.3390/ijms21217921. Int J Mol Sci. 2020. PMID: 33113841 Free PMC article.
-
Enzyme-catalyzed and binding reaction kinetics determined by titration calorimetry.Biochim Biophys Acta. 2016 May;1860(5):957-966. doi: 10.1016/j.bbagen.2015.12.018. Epub 2015 Dec 22. Biochim Biophys Acta. 2016. PMID: 26721335 Review.
-
Influence of cytosolic conditions on the reaction equilibrium and the reaction enthalpy of the enolase reaction accessed by calorimetry and van 't HOFF.Biochim Biophys Acta Gen Subj. 2020 Oct;1864(10):129675. doi: 10.1016/j.bbagen.2020.129675. Epub 2020 Jun 28. Biochim Biophys Acta Gen Subj. 2020. PMID: 32610157
-
Characterization of Enzymatic Reactions Using ITC.Methods Mol Biol. 2019;1964:251-266. doi: 10.1007/978-1-4939-9179-2_18. Methods Mol Biol. 2019. PMID: 30929248
-
A potential role for isothermal calorimetry in studies of the effects of thermodynamic non-ideality in enzyme-catalyzed reactions.J Mol Recognit. 2004 Sep-Oct;17(5):351-61. doi: 10.1002/jmr.706. J Mol Recognit. 2004. PMID: 15362092 Review.
Cited by
-
Indole-3-Carbinol Stabilizes p53 to Induce miR-34a, Which Targets LDHA to Block Aerobic Glycolysis in Liver Cancer Cells.Pharmaceuticals (Basel). 2022 Oct 13;15(10):1257. doi: 10.3390/ph15101257. Pharmaceuticals (Basel). 2022. PMID: 36297369 Free PMC article.
-
Thermodynamics and Kinetics of Glycolytic Reactions. Part II: Influence of Cytosolic Conditions on Thermodynamic State Variables and Kinetic Parameters.Int J Mol Sci. 2020 Oct 25;21(21):7921. doi: 10.3390/ijms21217921. Int J Mol Sci. 2020. PMID: 33113841 Free PMC article.
References
-
- Brown A.J. XXXVI.—Enzyme action. J. Chem. Soc. Perkin 1. 1902;81:373–388. doi: 10.1039/CT9028100373. - DOI
-
- Henri V. Lois Générales De L’action Des Diastases. Librairie Scientifique A.; Hermann, MO, USA: 1903.
-
- Henri V. Théorie Générale De L’action De Quelques Diastases. Gauthier-Villars; Paris, France: 1902.
-
- Michaelis L., Menton M.L. Kinetik der Invertinwirkung. Biochem. Z. 1913;49:333.
-
- Miller W.G., Alberty R.A. Kinetics of the reversible Michaelis-Menten mechanism and the applicability of the steady-state approximation. J. Am. Chem. Soc. 1958;80:5146–5151. doi: 10.1021/ja01552a034. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

