Review of PIP2 in Cellular Signaling, Functions and Diseases
- PMID: 33172190
- PMCID: PMC7664428
- DOI: 10.3390/ijms21218342
Review of PIP2 in Cellular Signaling, Functions and Diseases
Abstract
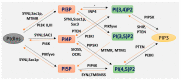
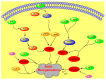
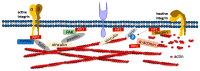
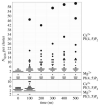
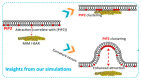
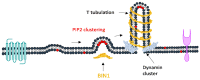
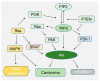
Phosphoinositides play a crucial role in regulating many cellular functions, such as actin dynamics, signaling, intracellular trafficking, membrane dynamics, and cell-matrix adhesion. Central to this process is phosphatidylinositol bisphosphate (PIP2). The levels of PIP2 in the membrane are rapidly altered by the activity of phosphoinositide-directed kinases and phosphatases, and it binds to dozens of different intracellular proteins. Despite the vast literature dedicated to understanding the regulation of PIP2 in cells over past 30 years, much remains to be learned about its cellular functions. In this review, we focus on past and recent exciting results on different molecular mechanisms that regulate cellular functions by binding of specific proteins to PIP2 or by stabilizing phosphoinositide pools in different cellular compartments. Moreover, this review summarizes recent findings that implicate dysregulation of PIP2 in many diseases.
Keywords: PIP2; Phosphoinositides; actin; diseases; focal adhesion; intracellular trafficking; membrane dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Downes C.P., Gray A., Lucocq J.M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. - PubMed
-
- Romarowski A., Battistone M.A., La Spina F.A., del Puga Molina L.C., Luque G.M., Vitale A.M., Cuasnicu P.S., Visconti P.E., Krapf D., Buffone M.G. PKA-dependent phosphorylation of LIMK1 and Cofilin is essential for mouse sperm acrosomal exocytosis. Dev. Biol. 2015;405:237–249. doi: 10.1016/j.ydbio.2015.07.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

