Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma
- PMID: 33172403
- PMCID: PMC7656738
- DOI: 10.1186/s12885-020-07503-y
Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma
Abstract
Background: For patients with multiple myeloma (MM), each additional line of therapy (LOT) is associated with lower response rates, shorter treatment duration and treatment-free intervals, and increased rates of toxicities and comorbidities. Here, we examine frontline treatment patterns, and attrition rates by LOT among newly diagnosed MM (NDMM) patients in the United States who were eligible or ineligible for autologous stem cell transplant (ASCT).
Methods: Data were identified from three US patient-level databases collectively covering the period January 2000 to September 2018. Patients had an index diagnosis of MM on or after January 1, 2007, medical and prescription insurance coverage at diagnosis, a 1-year look-back period prior to the index diagnosis, no prior malignancies in the 1-year period before index diagnosis, and had received ≥1 LOT.
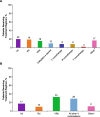
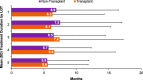
Results: Among patients who did not receive ASCT (non-transplant; n = 22,062), 12,557 (57%) received only 1 LOT and 9505 (43%) received > 1 LOT. Patients receiving only 1 LOT were significantly older, had higher mean Charlson Comorbidity Index (CCI) scores, and higher incidences of comorbidities. Among the 2763 patients receiving ASCT, 2184 received > 1 LOT, and 579 (21%) received only 1 LOT (ie, ASCT was the last treatment). 1682 (61%) patients received induction therapy as frontline treatment, of whom 187 (11%) also received consolidation therapy. The latter group was younger than those who received only induction therapy, had lower mean CCI scores, and comparable or lower incidences of selected comorbidities. The most common frontline therapy for non-transplant and transplant-eligible patients was bortezomib/dexamethasone and bortezomib/lenalidomide/dexamethasone, respectively. Attrition rates across all LOTs were high for non-transplant patients (range, 43-57%) and transplant patients (range, 21-37%). Treatment duration decreased by LOT for non-transplant patients and was consistent across LOTs for transplant patients.
Conclusions: In this analysis, a substantial proportion of patients with NDMM who received frontline therapy did not appear to receive a subsequent LOT. These high attrition rates underscore the need to use the most optimal treatment regimens upfront rather than reserving them for later LOTs in which the clinical benefit may decrease.
Keywords: Attrition rates; Autologous stem cell transplant; Bortezomib; Dexamethasone; Lenalidomide; Line of therapy; Newly diagnosed multiple myeloma; Treatment duration.
Conflict of interest statement
Rafael Fonseca served as a consultant for AbbVie, Aduro, Amgen, Bayer, BMS/Celgene, GlaxoSmithKline, Janssen, Juno, Kite, Merck, Novartis, Oncotracker, Pharmacyclics, and Sanofi; and participated in an advisory board for Adaptive Biotechnologies. Saad Z. Usmani served as a consultant/advisor for Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, SkylineDx, and Takeda; participated in a Speakers Bureau for Amgen, Celgene, Janssen, Sanofi, and Takeda; and received research grants from Amgen, Array Biopharma, Bristol-Myers Squibb, Celgene, Janssen, Merck, Pharmacyclics, Sanofi, and Takeda. Maneesha Mehra, Mary Slavcev, Jianming He, Sarah Cote, Annette Lam, Jon Ukropec, and Sandhya Nair are employees of Janssen. Eric M. Maiese was an employee of Janssen at the time the study was conducted. Ravi Potluri is an employee of SmartAnalyst Inc. Peter M. Voorhees received research funding from Amgen, Celgene, GlaxoSmithKline, Janssen, and Takeda; and served as a consultant/advisor for Adaptive Biotechnologies, Bristol-Myers Squibb, Celgene, Janssen, Novartis, Oncopeptides, Takeda, and TeneBio.
Figures
References
-
- SEER Cancer Stat Facts: Myeloma. Bethesda: National Cancer Institute. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed 24 Feb 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

