MET Exon 14-altered Lung Cancers and MET Inhibitor Resistance
- PMID: 33172896
- PMCID: PMC7854494
- DOI: 10.1158/1078-0432.CCR-20-2861
MET Exon 14-altered Lung Cancers and MET Inhibitor Resistance
Abstract
Purpose: MET tyrosine kinase inhibitors (TKIs) can achieve modest clinical outcomes in MET exon 14-altered lung cancers, likely secondary to primary resistance. Mechanisms of primary resistance remain poorly characterized and comprehensive proteomic analyses have not previously been performed.
Experimental design: We performed hybrid capture-based DNA sequencing, targeted RNA sequencing, cell-free DNA sequencing, selected reaction monitoring mass spectrometry (SRM-MS), and immunohistochemistry on patient samples of MET exon 14-altered lung cancers treated with a MET TKI. Associations between overall response rate (ORR), progression-free survival (PFS), and putative genomic alterations and MET protein expression were evaluated.
Results: Seventy-five of 168 MET exon 14-altered lung cancers received a MET TKI. Previously undescribed (zygosity, clonality, whole-genome duplication) and known (copy-number focality, tumor mutational burden, mutation region/type) genomic factors were not associated with ORR/PFS (P > 0.05). In contrast, MET expression was associated with MET TKI benefit. Only cases with detectable MET expression by SRM-MS (N = 15) or immunochemistry (N = 22) responded to MET TKI therapy, and cancers with H-score ≥ 200 had a higher PFS than cancers below this cutoff (10.4 vs. 5.5 months, respectively; HR, 3.87; P = 0.02).
Conclusions: In MET exon 14-altered cancers treated with a MET TKI, a comprehensive analysis of previously unknown and known genomic factors did not identify a genomic mechanism of primary resistance. Instead, MET expression correlated with benefit, suggesting the potential role of interrogating the proteome in addition to the genome in confirmatory prospective trials.
©2020 American Association for Cancer Research.
Figures


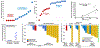
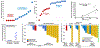

References
-
- KGaA M 2020. TEPMETKO (Tepotinib) Approved in Japan for Advanced NSCLC with METex14 Skipping Alterations. <https://www.emdgroup.com/en/news/tepotinib-25-03-2020.html>.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

