Inhibition of G-protein signalling in cardiac dysfunction of intellectual developmental disorder with cardiac arrhythmia (IDDCA) syndrome
- PMID: 33172956
- PMCID: PMC8639930
- DOI: 10.1136/jmedgenet-2020-107015
Inhibition of G-protein signalling in cardiac dysfunction of intellectual developmental disorder with cardiac arrhythmia (IDDCA) syndrome
Abstract
Background: Pathogenic variants of GNB5 encoding the β5 subunit of the guanine nucleotide-binding protein cause IDDCA syndrome, an autosomal recessive neurodevelopmental disorder associated with cognitive disability and cardiac arrhythmia, particularly severe bradycardia.
Methods: We used echocardiography and telemetric ECG recordings to investigate consequences of Gnb5 loss in mouse.
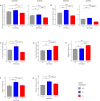
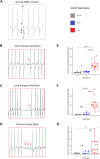
Results: We delineated a key role of Gnb5 in heart sinus conduction and showed that Gnb5-inhibitory signalling is essential for parasympathetic control of heart rate (HR) and maintenance of the sympathovagal balance. Gnb5-/- mice were smaller and had a smaller heart than Gnb5+/+ and Gnb5+/- , but exhibited better cardiac function. Lower autonomic nervous system modulation through diminished parasympathetic control and greater sympathetic regulation resulted in a higher baseline HR in Gnb5-/- mice. In contrast, Gnb5-/- mice exhibited profound bradycardia on treatment with carbachol, while sympathetic modulation of the cardiac stimulation was not altered. Concordantly, transcriptome study pinpointed altered expression of genes involved in cardiac muscle contractility in atria and ventricles of knocked-out mice. Homozygous Gnb5 loss resulted in significantly higher frequencies of sinus arrhythmias. Moreover, we described 13 affected individuals, increasing the IDDCA cohort to 44 patients.
Conclusions: Our data demonstrate that loss of negative regulation of the inhibitory G-protein signalling causes HR perturbations in Gnb5-/- mice, an effect mainly driven by impaired parasympathetic activity. We anticipate that unravelling the mechanism of Gnb5 signalling in the autonomic control of the heart will pave the way for future drug screening.
Keywords: GNB5variants; Gnb5-null mouse models; IDDCA; cardiac conduction anomalies.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Lodder EM, De Nittis P, Koopman CD, Wiszniewski W, Moura de Souza CF, Lahrouchi N, Guex N, Napolioni V, Tessadori F, Beekman L, Nannenberg EA, Boualla L, Blom NA, de Graaff W, Kamermans M, Cocciadiferro D, Malerba N, Mandriani B, Coban Akdemir ZH, Fish RJ, Eldomery MK, Ratbi I, Wilde AAM, de Boer T, Simonds WF, Neerman-Arbez M, Sutton VR, Kok F, Lupski JR, Reymond A, Bezzina CR, Bakkers J, Merla G. GNB5 mutations cause an autosomal-recessive multisystem syndrome with sinus bradycardia and cognitive disability. Am J Hum Genet 2016;99:786. 10.1016/j.ajhg.2016.08.011 - DOI - PMC - PubMed
-
- Shamseldin HE, Masuho I, Alenizi A, Alyamani S, Patil DN, Ibrahim N, Martemyanov KA, Alkuraya FS. GNB5 mutation causes a novel neuropsychiatric disorder featuring attention deficit hyperactivity disorder, severely impaired language development and normal cognition. Genome Biol 2016;17:195. 10.1186/s13059-016-1061-6 - DOI - PMC - PubMed
-
- Poke G, King C, Muir A, de Valles-Ibáñez G, Germano M, Moura de Souza CF, Fung J, Chung B, Fung CW, Mignot C, Ilea A, Keren B, Vermersch A-I, Davis S, Stanley T, Moharir M, Kannu P, Shao Z, Malerba N, Merla G, Mefford HC, Scheffer IE, Sadleir LG. The epileptology of GNB5 encephalopathy. Epilepsia 2019;60:e121–7. 10.1111/epi.16372 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G1001253/MRC_/Medical Research Council/United Kingdom
- R605/0717/DMT_/The Dunhill Medical Trust/United Kingdom
- G108/638/MRC_/Medical Research Council/United Kingdom
- WT093205 /WT_/Wellcome Trust/United Kingdom
- WT104033AIA/WT_/Wellcome Trust/United Kingdom
- MR/J004758/1/MRC_/Medical Research Council/United Kingdom
- G0802760/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_UP_1502/3/MRC_/Medical Research Council/United Kingdom
- MR/S005021/1/MRC_/Medical Research Council/United Kingdom
- G0601943/MRC_/Medical Research Council/United Kingdom
- MR/S01165X/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
