Lysosomal Biogenesis and Implications for Hydroxychloroquine Disposition
- PMID: 33172973
- PMCID: PMC7841421
- DOI: 10.1124/jpet.120.000309
Lysosomal Biogenesis and Implications for Hydroxychloroquine Disposition
Abstract
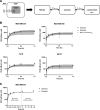
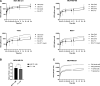
Lysosomes act as a cellular drug sink for weakly basic, lipophilic (lysosomotropic) xenobiotics, with many instances of lysosomal trapping associated with multiple drug resistance. Lysosomotropic agents have also been shown to activate master lysosomal biogenesis transcription factor EB (TFEB) and ultimately lysosomal biogenesis. We investigated the role of lysosomal biogenesis in the disposition of hydroxychloroquine (HCQ), a hallmark lysosomotropic agent, and observed that modulating the lysosomal volume of human breast cancer cell lines can account for differences in disposition of HCQ. Through use of an in vitro pharmacokinetic (PK) model, we characterized total cellular uptake of HCQ within the duration of static equilibrium (1 hour), as well as extended exposure to HCQ that is subject to dynamic equilibrium (>1 hour), wherein HCQ increases the size of the lysosomal compartment through swelling and TFEB-induced lysosomal biogenesis. In addition, we observe that pretreatment of cell lines with TFEB-activating agent Torin1 contributed to an increase of whole-cell HCQ concentrations by 1.4- to 1.6-fold, which were also characterized by the in vitro PK model. This investigation into the role of lysosomal volume dynamics in lysosomotropic drug disposition, including the ability of HCQ to modify its own disposition, advances our understanding of how chemically similar agents may distribute on the cellular level and examines a key area of lysosomal-mediated multiple drug resistance and drug-drug interaction. SIGNIFICANCE STATEMENT: Hydroxychloroquine is able to modulate its own cellular pharmacokinetic uptake by increasing the cellular lysosomal volume fraction through activation of lysosomal biogenesis master transcription factor EB and through lysosomal swelling. This concept can be applied to many other lysosomotropic drugs that activate transcription factor EB, such as doxorubicin and other tyrosine kinase inhibitor drugs, as these drugs may actively increase their own sequestration within the lysosome to further exacerbate multiple drug resistance and lead to potential acquired resistance.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare that no conflict of interest exists.
Figures



References
-
- Belhoussine R, Morjani H, Sharonov S, Ploton D, Manfait M. (1999) Characterization of intracellular pH gradients in human multidrug-resistant tumor cells by means of scanning microspectrofluorometry and dual-emission-ratio probes. Int J Cancer 81:81–89. - PubMed
-
- Burger H, den Dekker AT, Segeletz S, Boersma AW, de Bruijn P, Debiec-Rychter M, Taguchi T, Sleijfer S, Sparreboom A, Mathijssen RH, et al. (2015) Lysosomal sequestration determines intracellular imatinib levels. Mol Pharmacol 88:477–487. - PubMed
-
- Duvvuri M, Krise JP. (2005) A novel assay reveals that weakly basic model compounds concentrate in lysosomes to an extent greater than pH-partitioning theory would predict. Mol Pharm 2:440–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

