Magnesium Sensing Regulates Intestinal Colonization of Enterohemorrhagic Escherichia coli O157:H7
- PMID: 33173003
- PMCID: PMC7667037
- DOI: 10.1128/mBio.02470-20
Magnesium Sensing Regulates Intestinal Colonization of Enterohemorrhagic Escherichia coli O157:H7
Abstract
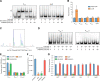
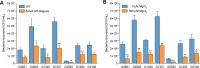
The large intestinal pathogen enterohemorrhagic Escherichia coli (EHEC) O157:H7 detects host cues to regulate virulence gene expression during colonization and infection. However, virulence regulatory mechanisms of EHEC O157:H7 in the human large intestine are not fully understood. Herein, we identified a virulence-regulating pathway where the PhoQ/PhoP two-component regulatory system senses low magnesium levels and signals to the O island 119-encoded Z4267 (LmiA; low magnesium-induced regulator A), directly activating loci of enterocyte effacement genes to promote EHEC O157:H7 adherence in the large intestine. Disruption of this pathway significantly decreased EHEC O157:H7 adherence in the mouse intestinal tract. Moreover, feeding mice a magnesium-rich diet significantly reduced EHEC O157:H7 adherence in vivo This LmiA-mediated virulence regulatory pathway is also conserved among several EHEC and enteropathogenic E. coli serotypes; therefore, our findings support the use of magnesium as a dietary supplement and provide greater insights into the dietary cues that can prevent enteric infections.IMPORTANCE Sensing specific gut metabolites is an important strategy for inducing crucial virulence programs by enterohemorrhagic Escherichia coli (EHEC) O157:H7 during colonization and infection. Here, we identified a virulence-regulating pathway wherein the PhoQ/PhoP two-component regulatory system signals to the O island 119-encoded low magnesium-induced regulator A (LmiA), which, in turn, activates locus of enterocyte effacement (LEE) genes to promote EHEC O157:H7 adherence in the low-magnesium conditions of the large intestine. This regulatory pathway is widely present in a range of EHEC and enteropathogenic E. coli (EPEC) serotypes. Disruption of this pathway significantly decreased EHEC O157:H7 adherence in the mouse intestinal tract. Moreover, mice fed a magnesium-rich diet showed significantly reduced EHEC O157:H7 adherence in vivo, indicating that magnesium may help in preventing EHEC and EPEC infection in humans.
Keywords: Z4267; bacterial adherence; gene regulation; locus of enterocyte effacement (LEE); magnesium; virulence.
Copyright © 2020 Liu et al.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

