Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells
- PMID: 33173147
- PMCID: PMC7655959
- DOI: 10.1038/s41598-020-76332-7
Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells
Abstract
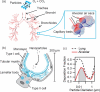
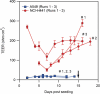
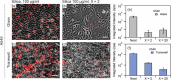
Pulmonary surfactant forms a sub-micrometer thick fluid layer that covers the surface of alveolar lumen and inhaled nanoparticles therefore come in to contact with surfactant prior to any interaction with epithelial cells. We investigate the role of the surfactant as a protective physical barrier by modeling the interactions using silica-Curosurf-alveolar epithelial cell system in vitro. Electron microscopy displays that the vesicles are preserved in the presence of nanoparticles while nanoparticle-lipid interaction leads to formation of mixed aggregates. Fluorescence microscopy reveals that the surfactant decreases the uptake of nanoparticles by up to two orders of magnitude in two models of alveolar epithelial cells, A549 and NCI-H441, irrespective of immersed culture on glass or air-liquid interface culture on transwell. Confocal microscopy corroborates the results by showing nanoparticle-lipid colocalization interacting with the cells. Our work thus supports the idea that pulmonary surfactant plays a protective role against inhaled nanoparticles. The effect of surfactant should therefore be considered in predictive assessment of nanoparticle toxicity or drug nanocarrier uptake. Models based on the one presented in this work may be used for preclinical tests with engineered nanoparticles.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Weagle CL, et al. Global sources of fine particulate matter: interpretation of PM2.5 chemical composition observed by SPARTAN using a global chemical transport model. Environ. Sci. Technol. 2018;52:11670–11681. - PubMed
-
- Hofmann W. Modelling inhaled particle deposition in the human lung—a review. J. Aerosol Sci. 2011;42:693–724. doi: 10.1016/j.jaerosci.2011.05.007. - DOI
-
- Notter RH. Lung Surfactant: Basic Science and Clinical Applications. Boca Raton: CRC Press; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

