Optically Generated Ultrasound for Intracoronary Imaging
- PMID: 33173786
- PMCID: PMC7591717
- DOI: 10.3389/fcvm.2020.525530
Optically Generated Ultrasound for Intracoronary Imaging
Abstract
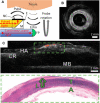
Conventional intravascular ultrasound (IVUS) devices use piezoelectric transducers to electrically generate and receive US. With this paradigm, there are numerous challenges that restrict improvements in image quality. First, with miniaturization of the transducers to reduce device size, it can be challenging to achieve the sensitivities and bandwidths required for large tissue penetration depths and high spatial resolution. Second, complexities associated with manufacturing miniaturized electronic transducers can have significant cost implications. Third, with increasing interest in molecular characterization of tissue in-vivo, it has been challenging to incorporate optical elements for multimodality imaging with photoacoustics (PA) or near-infrared spectroscopy (NIRS) whilst maintaining the lateral dimensions suitable for intracoronary imaging. Optical Ultrasound (OpUS) is a new paradigm for intracoronary imaging. US is generated at the surface of a fiber optic transducer via the photoacoustic effect. Pulsed or modulated light is absorbed in an engineered coating on the fiber surface and converted to thermal energy. The subsequent temperature rise leads to a pressure rise within the coating, which results in a propagating ultrasound wave. US reflections from imaged structures are received with optical interferometry. With OpUS, high bandwidths (31.5 MHz) and pressures (21.5 MPa) have enabled imaging with axial resolutions better than 50 μm and at depths >20 mm. These values challenge those of conventional 40 MHz IVUS technology and show great potential for future clinical application. Recently developed nanocomposite coating materials, that are highly transmissive at light wavelengths used for PA and NIRS light, can facilitate multimodality imaging, thereby enabling molecular characterization.
Keywords: IVUS; OPUS; imaging; intravascular ultrasound; optical ultrasound; optoacoustics.
Copyright © 2020 Little, Colchester, Noimark, Manmathan, Finlay, Desjardins and Rakhit.
Figures

References
-
- Waksman R, Di Mario C, Torguson R, Ali ZA, Singh V, Skinner WH, et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet. (2019) 294:1629–37. 10.1016/S0140-6736(19)31794-5 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

