Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study
- PMID: 33173853
- PMCID: PMC7644240
- DOI: 10.1016/j.eclinm.2020.100590
Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study
Abstract
Background: Complement pathway inhibition may provide benefit for severe acute respiratory illnesses caused by viral infections such as COVID-19. We present results from a nonrandomized proof-of-concept study of complement C5 inhibitor eculizumab for treatment of severe COVID-19.
Methods: All patients (N = 80) with confirmed SARS-CoV-2 infection and severe COVID-19 admitted to our intensive care unit between March 10 and May 5, 2020 were included. Forty-five patients were treated with standard care and 35 with standard care plus eculizumab through expanded-access emergency treatment. The prespecified primary outcome was day-15 survival. Clinical laboratory values and biomarkers, complement levels, and treatment-emergent serious adverse events (TESAEs) were also assessed.
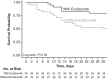
Findings: At day 15, estimated survival was 82.9% (95% CI: 70.4%‒95.3%) with eculizumab and 62.2% (48.1%‒76.4%) without eculizumab (log-rank test, P = 0.04). Patients treated with eculizumab experienced a significantly more rapid decrease in lactate, blood urea nitrogen, total and conjugated bilirubin levels and a significantly more rapid increase in platelet count, prothrombin time, and in the ratio of arterial oxygen tension over fraction of inspired oxygen versus patients treated without eculizumab. Eculizumab-associated changes in complement levels, laboratory values, and biomarkers were consistent with terminal complement inhibition, reduced hypoxia, and decreased inflammation. TESAEs of special interest occurring in >5% of patients treated with/without eculizumab were ventilator-associated pneumonia (51%/24%), bacteremia (11%/2%), gastroduodenal hemorrhage (14%/16%), and hemolysis (3%/18%).
Interpretation: Findings from this proof-of-concept study suggest eculizumab may improve survival and reduce hypoxia in patients with severe COVID-19. Randomized studies evaluating the efficacy and safety of this treatment approach are needed.
Funding: Programme d'Investissements d'Avenir: ANR-18-RHUS60004.
Keywords: Acute respiratory distress syndrome; C5 inhibitor; Complement pathway; Coronavirus; Cytokines; Pneumonia; Sepsis.
© 2020 The Author(s).
Conflict of interest statement
Djillali Annane: reports non-financial support from Alexion Pharmaceuticals Inc. and grants from Programme d'Investissements d'Avenir during the conduct of the study; other (investigator) from Alexion outside the submitted work. Nicholas Heming: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study; other (investigator) from Alexion outside the submitted work. Lamiae Grimaldi-Bensouda: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study. Véronique Frémeaux-Bacchi: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study; grants and personal fees from Alexion, personal fees from Roche, personal fees from Apellis, and personal fees from Biocryps outside the submitted work. Marie Vigan: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study. Anne-Laure Roux: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study. Armance Marchal: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study. Hugues Michelon: reports non-financial support from Alexion Pharmaceuticals Inc, during the conduct of the study. Martin Rottman: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study. Pierre Moine: reports non-financial support from Alexion Pharmaceuticals Inc. during the conduct of the study; non-financial support and other (investigator) from Alexion outside the submitted work.
Figures

References
-
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Available at: https://coronavirus.jhu.edu/map.html. Accessed June 9, 2020.
-
- US Centers for Disease Control and Prevention. COVIDView: a weekly surveillance summary of U.S. COVID-19 Activity, key updates for week 12, ending May 30, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed June 9, 2020.
-
- Santé Publique France. Infection au nouveau Coronavirus (SARS-CoV-2), COVID-19, France et Monde. Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-.... Accessed June 9, 2020.
LinkOut - more resources
Full Text Sources
Miscellaneous

