This is a preprint.
Intranasal fusion inhibitory lipopeptide prevents direct contact SARS-CoV-2 transmission in ferrets
- PMID: 33173865
- PMCID: PMC7654853
- DOI: 10.1101/2020.11.04.361154
Intranasal fusion inhibitory lipopeptide prevents direct contact SARS-CoV-2 transmission in ferrets
Update in
-
Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets.Science. 2021 Mar 26;371(6536):1379-1382. doi: 10.1126/science.abf4896. Epub 2021 Feb 17. Science. 2021. PMID: 33597220 Free PMC article.
Abstract
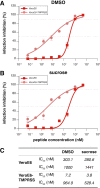
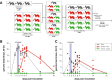
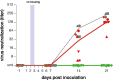
Containment of the COVID-19 pandemic requires reducing viral transmission. SARS-CoV-2 infection is initiated by membrane fusion between the viral and host cell membranes, mediated by the viral spike protein. We have designed a dimeric lipopeptide fusion inhibitor that blocks this critical first step of infection for emerging coronaviruses and document that it completely prevents SARS-CoV-2 infection in ferrets. Daily intranasal administration to ferrets completely prevented SARS-CoV-2 direct-contact transmission during 24-hour co-housing with infected animals, under stringent conditions that resulted in infection of 100% of untreated animals. These lipopeptides are highly stable and non-toxic and thus readily translate into a safe and effective intranasal prophylactic approach to reduce transmission of SARS-CoV-2.
One-sentence summary: A dimeric form of a SARS-CoV-2-derived lipopeptide is a potent inhibitor of fusion and infection in vitro and transmission in vivo .
Conflict of interest statement
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
