This is a preprint.
Viral protein engagement of GBF1 induces host cell vulnerability through synthetic lethality
- PMID: 33173868
- PMCID: PMC7654857
- DOI: 10.1101/2020.10.12.336487
Viral protein engagement of GBF1 induces host cell vulnerability through synthetic lethality
Update in
-
Viral protein engagement of GBF1 induces host cell vulnerability through synthetic lethality.J Cell Biol. 2022 Nov 7;221(11):e202011050. doi: 10.1083/jcb.202011050. Epub 2022 Oct 28. J Cell Biol. 2022. PMID: 36305789 Free PMC article.
Abstract
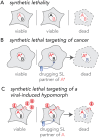
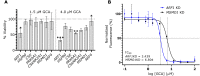
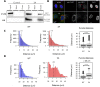
Viruses co-opt host proteins to carry out their lifecycle. Repurposed host proteins may thus become functionally compromised; a situation analogous to a loss-of-function mutation. We term such host proteins viral-induced hypomorphs. Cells bearing cancer driver loss-of-function mutations have successfully been targeted with drugs perturbing proteins encoded by the synthetic lethal partners of cancer-specific mutations. Synthetic lethal interactions of viral-induced hypomorphs have the potential to be similarly targeted for the development of host-based antiviral therapeutics. Here, we use GBF1, which supports the infection of many RNA viruses, as a proof-of-concept. GBF1 becomes a hypomorph upon interaction with the poliovirus protein 3A. Screening for synthetic lethal partners of GBF1 revealed ARF1 as the top hit, disruption of which, selectively killed cells that synthesize poliovirus 3A. Thus, viral protein interactions can induce hypomorphs that render host cells vulnerable to perturbations that leave uninfected cells intact. Exploiting viral-induced vulnerabilities could lead to broad-spectrum antivirals for many viruses, including SARS-CoV-2.
Summary: Using a viral-induced hypomorph of GBF1, Navare et al., demonstrate that the principle of synthetic lethality is a mechanism to selectively kill virus-infected cells.
Figures


References
-
- Beijersbergen R.L., Wessels L.F.A., and Bernards R.. 2017. Synthetic lethality in cancer therapeutics. Annu. Rev. Cancer Biol. doi: 10.1146/annurev-cancerbio-042016-073434 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
