Molecular mechanisms of cancer cachexia‑induced muscle atrophy (Review)
- PMID: 33174001
- PMCID: PMC7646947
- DOI: 10.3892/mmr.2020.11608
Molecular mechanisms of cancer cachexia‑induced muscle atrophy (Review)
Abstract
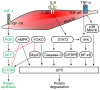
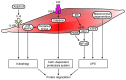
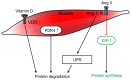
Muscle atrophy is a severe clinical problem involving the loss of muscle mass and strength that frequently accompanies the development of numerous types of cancer, including pancreatic, lung and gastric cancers. Cancer cachexia is a multifactorial syndrome characterized by a continuous decline in skeletal muscle mass that cannot be reversed by conventional nutritional therapy. The pathophysiological characteristic of cancer cachexia is a negative protein and energy balance caused by a combination of factors, including reduced food intake and metabolic abnormalities. Numerous necessary cellular processes are disrupted by the presence of abnormal metabolites, which mediate several intracellular signaling pathways and result in the net loss of cytoplasm and organelles in atrophic skeletal muscle during various states of cancer cachexia. Currently, the clinical morbidity and mortality rates of patients with cancer cachexia are high. Once a patient enters the cachexia phase, the consequences are difficult to reverse and the treatment methods for cancer cachexia are very limited. The present review aimed to summarize the recent discoveries regarding the pathogenesis of cancer cachexia‑induced muscle atrophy and provided novel ideas for the comprehensive treatment to improve the prognosis of affected patients.
Figures
References
-
- Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, Fearon K, Strasser F, Kaasa S; Euro-Impact. Validation of the consensus-definition for cancer cachexia and evaluation of a classification model-a study based on data from an international multicentre project (EPCRC-CSA) Ann Oncol. 2014;25:1635–1642. doi: 10.1093/annonc/mdu086. - DOI - PubMed
-
- Warnold I, Lundholm K, Schersten T. Energy balance and body composition in cancer patients. Cancer Res. 1978;38:1801–1807. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

