Updated Characterization of Outbreak Response Strategies for 2019-2029: Impacts of Using a Novel Type 2 Oral Poliovirus Vaccine Strain
- PMID: 33174263
- PMCID: PMC7887065
- DOI: 10.1111/risa.13622
Updated Characterization of Outbreak Response Strategies for 2019-2029: Impacts of Using a Novel Type 2 Oral Poliovirus Vaccine Strain
Abstract
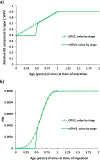
Delays in achieving the global eradication of wild poliovirus transmission continue to postpone subsequent cessation of all oral poliovirus vaccine (OPV) use. Countries must stop OPV use to end all cases of poliomyelitis, including vaccine-associated paralytic polio (VAPP) and cases caused by vaccine-derived polioviruses (VDPVs). The Global Polio Eradication Initiative (GPEI) coordinated global cessation of all type 2 OPV (OPV2) use in routine immunization in 2016 but did not successfully end the transmission of type 2 VDPVs (VDPV2s), and consequently continues to use type 2 OPV (OPV2) for outbreak response activities. Using an updated global poliovirus transmission and OPV evolution model, we characterize outbreak response options for 2019-2029 related to responding to VDPV2 outbreaks with a genetically stabilized novel OPV (nOPV2) strain or with the currently licensed monovalent OPV2 (mOPV2). Given uncertainties about the properties of nOPV2, we model different assumptions that appear consistent with the evidence on nOPV2 to date. Using nOPV2 to respond to detected cases may reduce the expected VDPV and VAPP cases and the risk of needing to restart OPV2 use in routine immunization compared to mOPV2 use for outbreak response. The actual properties, availability, and use of nOPV2 will determine its effects on type 2 poliovirus transmission in populations. Even with optimal nOPV2 performance, countries and the GPEI would still likely need to restart OPV2 use in routine immunization in OPV-using countries if operational improvements in outbreak response to stop the transmission of cVDPV2s are not implemented effectively.
Keywords: Dynamic modeling; outbreak response; polio.
© 2020 Society for Risk Analysis.
Figures




References
-
- Bandyopadhyay AS (2020a). Clinical data from novel type-2 oral polio vaccine trials and plan for emergency use listing. Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization, March – April 2020 Retrieved from https://www.who.int/immunization/sage/meetings/2019/october/Bandyopadhya...
-
- Bandyopadhyay AS (2020b). Summary of nOPV2 clinical data. Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization, March – April 2020 Retrieved from https://www.who.int/immunization/sage/meetings/2020/april/SAGE_Slidedeck...
-
- Brown MC, Holl EK, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, … Nair SK (2017). Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen–specific CTLs. Sci Transl Med, 9(408), eaan4220 10.1126/scitranslmed.aan4220 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

