LHH1, a novel antimicrobial peptide with anti-cancer cell activity identified from Lactobacillus casei HZ1
- PMID: 33175275
- PMCID: PMC7658291
- DOI: 10.1186/s13568-020-01139-8
LHH1, a novel antimicrobial peptide with anti-cancer cell activity identified from Lactobacillus casei HZ1
Abstract
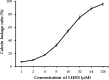
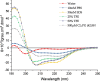
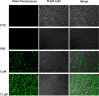
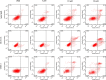
Antimicrobial peptides have been attracting increasing attention for their multiple beneficial effects. In present study, a novel AMP with a molecular weight of 1875.5 Da, was identified from the genome of Lactobacillus casei HZ1. The peptide, which was named as LHH1 was comprised of 16 amino acid residues, and its α-helix content was 95.34% when dissolved in 30 mM SDS. LHH1 exhibited a broad range of antimicrobial activities against Gram-positive bacteria and fungus. It could effectively inhibit Staphylococcus aureus with a minimum inhibitory concentration of 3.5 μM and showed a low hemolytic activity. The scanning electron microscope, confocal laser scanning microscope and flow cytometry results showed that LHH1 exerted its antibacterial activity by damaging the cell membrane of Staphylococcus aureus. Meanwhile, LHH1 also exhibited anti-cancer cell activities against several cancer cells via breaking the cell membrane of MGC803, HCT116 and C666-1 cancer cells.
Keywords: Anti-cancer; Antimicrobial peptide; Lactobacillus casei; Pathogenic bacteria; Staphylococcus aureus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Identification, Recombinant Expression, and Characterization of LHG2, a Novel Antimicrobial Peptide of Lactobacillus casei HZ1.Molecules. 2018 Sep 3;23(9):2246. doi: 10.3390/molecules23092246. Molecules. 2018. PMID: 30177656 Free PMC article.
-
Anti-proliferative effect on a colon adenocarcinoma cell line exerted by a membrane disrupting antimicrobial peptide KL15.Cancer Biol Ther. 2015;16(8):1172-83. doi: 10.1080/15384047.2015.1056407. Cancer Biol Ther. 2015. PMID: 26147829 Free PMC article.
-
Probiotic Lactobacillus casei expressing porcine antimicrobial peptide PR39 elevates antibacterial activity in the gastrointestinal tract.Can J Microbiol. 2016 Nov;62(11):961-969. doi: 10.1139/cjm-2016-0130. Epub 2016 Jul 7. Can J Microbiol. 2016. PMID: 27718591
-
Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus.AMB Express. 2019 Jul 30;9(1):122. doi: 10.1186/s13568-019-0843-0. AMB Express. 2019. PMID: 31363941 Free PMC article.
-
Effective antimicrobial activity of a peptide mutant Cbf-14-2 against penicillin-resistant bacteria based on its unnatural amino acids.Eur J Pharm Sci. 2017 Jul 15;105:169-177. doi: 10.1016/j.ejps.2017.05.030. Epub 2017 May 15. Eur J Pharm Sci. 2017. PMID: 28522372
Cited by
-
Antimicrobial peptides and their potential application in antiviral coating agents.Colloids Surf B Biointerfaces. 2022 Sep;217:112693. doi: 10.1016/j.colsurfb.2022.112693. Epub 2022 Jul 8. Colloids Surf B Biointerfaces. 2022. PMID: 35853393 Free PMC article. Review.
-
Probiotic-Derived Bioactive Compounds in Colorectal Cancer Treatment.Microorganisms. 2023 Jul 27;11(8):1898. doi: 10.3390/microorganisms11081898. Microorganisms. 2023. PMID: 37630458 Free PMC article. Review.
-
An Update on the Effectiveness of Probiotics in the Prevention and Treatment of Cancer.Life (Basel). 2022 Jan 2;12(1):59. doi: 10.3390/life12010059. Life (Basel). 2022. PMID: 35054452 Free PMC article. Review.
-
Selective Induction of Intrinsic Apoptosis in Retinoblastoma Cells by Novel Cationic Antimicrobial Dodecapeptides.Pharmaceutics. 2022 Nov 18;14(11):2507. doi: 10.3390/pharmaceutics14112507. Pharmaceutics. 2022. PMID: 36432697 Free PMC article.
-
Bacteriocins in Cancer Treatment: Mechanisms and Clinical Potentials.Biomolecules. 2024 Jul 10;14(7):831. doi: 10.3390/biom14070831. Biomolecules. 2024. PMID: 39062544 Free PMC article. Review.
References
-
- Ahn HS, Cho W, Kang SH, Ko SS, Park MS, Cho H, Lee KH. Design and synthesis of novel antimicrobial peptides on the basis of alpha helical domain of Tenecin 1, an insect defensin protein, and structure-activity relationship study. Peptides. 2006;27(4):640–648. doi: 10.1016/j.peptides.2005.08.016. - DOI - PubMed
-
- Arpornsuwan T, Sriwai W, Jaresitthikunchai J, Phaonakrop N, Sritanaudomchai H, Roytrakul S. Anticancer activities of antimicrobial BmKn2 peptides against oral and colon cancer cells. Int J Pept Res Ther. 2014;20(4):501–509. doi: 10.1007/s10989-014-9417-9. - DOI
Grants and funding
- 2017YFD0400303/National Key R&D Program of China
- 18JCZDJC33800/Natural Science Foundation of Tianjin
- TD13-5015/Innovative Research Team of Tianjin Municipral Education Commission
- 2016LG06/Young Teachers' Innovation Fund of Tianjin University of Science and Technology
- 17PTGCCX00190/Project for the Public Service Platform of Strain Breeding and Fermentation Technology of Industrial Microorganisms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

