Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives
- PMID: 33175307
- PMCID: PMC7657088
- DOI: 10.1007/s11626-020-00517-7
Invited review: human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives
Abstract
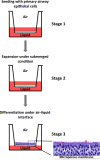
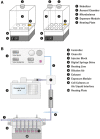
The lung is an organ that is directly exposed to the external environment. Given the large surface area and extensive ventilation of the lung, it is prone to exposure to airborne substances, such as pathogens, allergens, chemicals, and particulate matter. Highly elaborate and effective mechanisms have evolved to protect and maintain homeostasis in the lung. Despite these sophisticated defense mechanisms, the respiratory system remains highly susceptible to environmental challenges. Because of the impact of respiratory exposure on human health and disease, there has been considerable interest in developing reliable and predictive in vitro model systems for respiratory toxicology and basic research. Human air-liquid-interface (ALI) organotypic airway tissue models derived from primary tracheobronchial epithelial cells have in vivo-like structure and functions when they are fully differentiated. The presence of the air-facing surface allows conducting in vitro exposures that mimic human respiratory exposures. Exposures can be conducted using particulates, aerosols, gases, vapors generated from volatile and semi-volatile substances, and respiratory pathogens. Toxicity data have been generated using nanomaterials, cigarette smoke, e-cigarette vapors, environmental airborne chemicals, drugs given by inhalation, and respiratory viruses and bacteria. Although toxicity evaluations using human airway ALI models require further standardization and validation, this approach shows promise in supplementing or replacing in vivo animal models for conducting research on respiratory toxicants and pathogens.
Keywords: Air-liquid-interface (ALI) airway cultures; Exposure system; Inhalation toxicology; Pathogen-host interaction; Pulmonary drug testing.
Figures

References
-
- Adamson J, Li X, Cui H, Thorne D, Xie F, Gaca MD. Nicotine quantification in vitro: a consistent dosimetry marker for e-cigarette aerosol and cigarette smoke generation. Appl In Vitro Toxicol. 2017;3:14–27. doi: 10.1089/aivt.2016.0025. - DOI
-
- Adamson J, Thorne D, Errington G, Fields W, Li X, Payne R, Krebs T, Dalrymple A, Fowler K, Dillon D, Xie F, Meredith C. An inter-machine comparison of tobacco smoke particle deposition in vitro from six independent smoke exposure systems. Toxicol in Vitro. 2014;28:1320–1328. doi: 10.1016/j.tiv.2014.06.012. - DOI - PubMed
-
- Adler KB, Schwarz JE, Whitcutt MJ, Wu R. A new chamber system for maintaining differentiated Guinea pig respiratory epithelial cells between air and liquid phases. Biotechniques. 1987;5:462–465.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

