On the treatment effect heterogeneity of antidepressants in major depression: A Bayesian meta-analysis and simulation study
- PMID: 33175895
- PMCID: PMC7657525
- DOI: 10.1371/journal.pone.0241497
On the treatment effect heterogeneity of antidepressants in major depression: A Bayesian meta-analysis and simulation study
Abstract
Background: The average treatment effect of antidepressants in major depression was found to be about 2 points on the 17-item Hamilton Depression Rating Scale, which lies below clinical relevance. Here, we searched for evidence of a relevant treatment effect heterogeneity that could justify the usage of antidepressants despite their low average treatment effect.
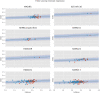
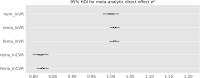
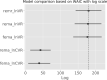
Methods: Bayesian meta-analysis of 169 randomized, controlled trials including 58,687 patients. We considered the effect sizes log variability ratio (lnVR) and log coefficient of variation ratio (lnCVR) to analyze the difference in variability of active and placebo response. We used Bayesian random-effects meta-analyses (REMA) for lnVR and lnCVR and fitted a random-effects meta-regression (REMR) model to estimate the treatment effect variability between antidepressants and placebo.
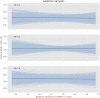
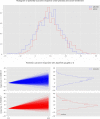
Results: The variability ratio was found to be very close to 1 in the best fitting models (REMR: 95% highest density interval (HDI) [0.98, 1.02], REMA: 95% HDI [1.00, 1.02]). The between-study standard deviation τ under the REMA with respect to lnVR was found to be low (95% HDI [0.00, 0.02]). Simulations showed that a large treatment effect heterogeneity is only compatible with the data if a strong correlation between placebo response and individual treatment effect is assumed.
Conclusions: The published data from RCTs on antidepressants for the treatment of major depression is compatible with a near-constant treatment effect. Although it is impossible to rule out a substantial treatment effect heterogeneity, its existence seems rather unlikely. Since the average treatment effect of antidepressants falls short of clinical relevance, the current prescribing practice should be re-evaluated.
Conflict of interest statement
CAM received consulting fees from Silence Therapeutics, outside the submitted work. The other authors declared no competing interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates. https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017....
-
- DGPPN. S3-Leitlinie und Nationale VersorgungsLeitlinie (NVL) Unipolare Depression, 2. Auflage. 2015. https://www.leitlinien.de/mdb/downloads/nvl/depression/depression-2aufl-....
-
- NICE. Depression in adults: recognition and management. 2009. https://www.nice.org.uk/guidance/cg90/chapter/1-Guidance#treatment-choic.... - PubMed
-
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet [Internet]. 2018. April 7;391(10128):1357–66. Available from: 10.1016/S0140-6736(17)32802-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

