Long-Term Glycaemic Durability of Early Combination Therapy Strategy versus Metformin Monotherapy in Korean Patients with Newly Diagnosed Type 2 Diabetes Mellitus
- PMID: 33176094
- PMCID: PMC8640145
- DOI: 10.4093/dmj.2020.0173
Long-Term Glycaemic Durability of Early Combination Therapy Strategy versus Metformin Monotherapy in Korean Patients with Newly Diagnosed Type 2 Diabetes Mellitus
Abstract
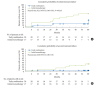
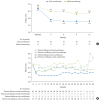
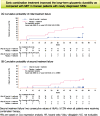
We assessed the glycaemic durability with early combination (EC; vildagliptin+metformin [MET], n=22) versus MET monotherapy (n=17), among newly-diagnosed type 2 diabetes mellitus (T2DM) enrolled (between 2012 and 2014) in the VERIFY study from Korea (n=39). Primary endpoint was time to initial treatment failure (TF) (glycosylated hemoglobin [HbA1c] ≥7.0% at two consecutive scheduled visits after randomization [end of period 1]). Time to second TF was assessed when both groups were receiving and failing on the combination (end of period 2). With EC the risk of initial TF significantly reduced by 78% compared to MET (n=3 [15%] vs. n=10 [58.7%], P=0.0228). No secondary TF occurred in EC group versus five patients (29.4%) in MET. Patients receiving EC treatment achieved consistently lower HbA1c levels. Both treatment approaches were well tolerated with no hypoglycaemic events. In Korean patients with newly diagnosed T2DM, EC treatment significantly and consistently improved the long-term glycaemic durability as compared with MET.
Keywords: Diabetes mellitus, type 2; Drug therapy, combination; Glycated hemoglobin A; Korea; Metformin; Vildagliptin.
Conflict of interest statement
Soon-Jib Yoo, Sang-Ah Chang, Tae Seo Sohn, Hyuk-Sang Kwon, Jong Min Lee, and Sungdae Moon have nothing to disclose. Pieter Proot is employed by and own stocks in Novartis. Päivi M. Paldánius was the medical lead of the VERIFY study and employed by Novartis Pharma AG at and up to the time of study completion. Kun Ho Yoon has served as a consultant for Novo Nordisk and MSD; received honorarium as a speaker from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, MSD, Novo Nordisk, Sanofi, and Takeda; and received research support from AstraZeneca and Takeda.
Figures
References
-
- nanditha a, ma rc, ramachandran a, snehalatha c, chan jc, chia ks, et al. diabetes in asia and the pacific: implications for the global epidemic. diabetes care. 2016;39:472–85. - PubMed
-
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. - PubMed
-
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–701. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

