Capturing Cardiogenesis in Gastruloids
- PMID: 33176168
- PMCID: PMC7867643
- DOI: 10.1016/j.stem.2020.10.013
Capturing Cardiogenesis in Gastruloids
Abstract
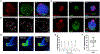
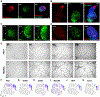
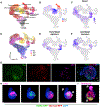
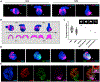
Organoids are powerful models for studying tissue development, physiology, and disease. However, current culture systems disrupt the inductive tissue-tissue interactions needed for the complex morphogenetic processes of native organogenesis. Here, we show that mouse embryonic stem cells (mESCs) can be coaxed to robustly undergo fundamental steps of early heart organogenesis with an in-vivo-like spatiotemporal fidelity. These axially patterned embryonic organoids (gastruloids) mimic embryonic development and support the generation of cardiovascular progenitors, including first and second heart fields. The cardiac progenitors self-organize into an anterior domain reminiscent of a cardiac crescent before forming a beating cardiac tissue near a putative primitive gut-like tube, from which it is separated by an endocardial-like layer. These findings unveil the surprising morphogenetic potential of mESCs to execute key aspects of organogenesis through the coordinated development of multiple tissues. This platform could be an excellent tool for studying heart development in unprecedented detail and throughput.
Keywords: 3D cardiac tissue; cardiac organoid; cardiogenesis; development; embryonic organoids; heart; in vitro organogenesis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A.B. is part of Viventis Microscopy Sàrl that has commercialized the LS1 light-sheet microscope used in this study for time-lapse imaging of gastruloids. The EPFL (with Cambridge Enterprise Limited) has filed for patent protection (PCT/GB2019/052668) on the embryoid technology described herein, and M.P.L. and G.R. are named as inventors on the patent.
Figures

Comment in
-
Mouse gastruloids take heart.Nat Rev Cardiol. 2021 Apr;18(4):233-234. doi: 10.1038/s41569-020-00501-4. Nat Rev Cardiol. 2021. PMID: 33414560 No abstract available.
-
Taking Heart Development to the Next Level.Cell Stem Cell. 2021 Feb 4;28(2):180-181. doi: 10.1016/j.stem.2021.01.014. Cell Stem Cell. 2021. PMID: 33545075
-
Recapitulating early cardiogenesis in vitro.Nat Methods. 2021 Apr;18(4):331. doi: 10.1038/s41592-021-01118-2. Nat Methods. 2021. PMID: 33828266 No abstract available.
-
Follow your heart and trust your gut: Co-development of heart and gut tissue in organoids.Cell Stem Cell. 2021 Dec 2;28(12):2037-2038. doi: 10.1016/j.stem.2021.09.003. Cell Stem Cell. 2021. PMID: 34861142
References
-
- Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy A-C, Lutolf MP, Duboule D, and Arias AM (2018). Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

