High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa
- PMID: 33176202
- PMCID: PMC7648883
- DOI: 10.1016/j.ijid.2020.10.104
High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa
Abstract
Objective: Significant morbidity and mortality have occurred in the USA, Europe, and Asia due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), whereas the numbers of infections and deaths in sub-Saharan Africa (SSA) have remained comparatively low. It has been hypothesized that exposure of the population in SSA to other coronaviruses prior to the COVID-19 pandemic resulted in some degree of cross-protection against SARS-CoV-2 infection and pathogenesis. We evaluated this hypothesis by comparing SARS-CoV-2 cross-reactive antibodies in pre-pandemic plasma samples collected from SSA and the USA.
Method: Pre-COVID-19 pandemic plasma samples from SSA and the USA were collected and tested by immunofluorescence assay against the spike and nucleocapsid proteins of all known human coronaviruses (HCoVs).
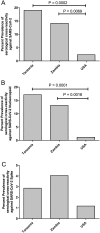
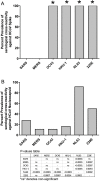
Results: The prevalence of SARS-CoV-2 serological cross-reactivity was significantly higher in samples from SSA compared with the USA. Most of these cross-reactive samples cross-recognized the SARS-CoV-2 nucleocapsid protein and the spike proteins of other HCoVs. Nucleocapsid proteins from HCoV-NL63 and HCoV-229E were detected in most samples, thereby implicating prior exposure to these two HCoVs as the likely source of cross-reactive antibodies against SARS-CoV-2.
Conclusion: The low incidences of SARS-CoV-2 infection and disease in SSA appear to be correlated with the pre-pandemic serological cross-recognition of HCoVs, which are substantially more prevalent in SSA than the USA.
Keywords: COVID-19; Cross-reactivity; HCoV-229E; HCoV-NL63; Human coronavirus; SARS-CoV-2; Serology; Sub-Saharan Africa.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Boni M.F., Lemey P., Jiang X., Lam T.T., Perry B.W., Castoe T.A. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. 2020;5:1408–1417. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

