Physiomimetic Models of Adenomyosis
- PMID: 33176387
- PMCID: PMC7803459
- DOI: 10.1055/s-0040-1719084
Physiomimetic Models of Adenomyosis
Abstract
Adenomyosis remains an enigmatic disease in the clinical and research communities. The high prevalence, diversity of morphological and symptomatic presentations, array of potential etiological explanations, and variable response to existing interventions suggest that different subgroups of patients with distinguishable mechanistic drivers of disease may exist. These factors, combined with the weak links to genetic predisposition, make the entire spectrum of the human condition challenging to model in animals. Here, after an overview of current approaches, a vision for applying physiomimetic modeling to adenomyosis is presented. Physiomimetics combines a system's biology analysis of patient populations to generate hypotheses about mechanistic bases for stratification with in vitro patient avatars to test these hypotheses. A substantial foundation for three-dimensional (3D) tissue engineering of adenomyosis lesions exists in several disparate areas: epithelial organoid technology; synthetic biomaterials matrices for epithelial-stromal coculture; smooth muscle 3D tissue engineering; and microvascular tissue engineering. These approaches can potentially be combined with microfluidic platform technologies to model the lesion microenvironment and can potentially be coupled to other microorgan systems to examine systemic effects. In vitro patient-derived models are constructed to answer specific questions leading to target identification and validation in a manner that informs preclinical research and ultimately clinical trial design.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
None declared.
Figures
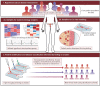



References
-
- Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98(03):572–579. - PubMed
-
- Chapron C, Tosti C, Marcellin L. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod. 2017;32(07):1393–1401. - PubMed
-
- García-Solares J, Donnez J, Donnez O, Dolmans M M. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(03):371–379. - PubMed
-
- Habiba M, Gordts S, Bazot M, Brosens I, Benagiano G. Exploring the challenges for a new classification of adenomyosis. Reprod Biomed Online. 2020;40(04):569–581. - PubMed

