Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document
- PMID: 33176455
- PMCID: PMC7673642
- DOI: 10.1161/CIRCHEARTFAILURE.120.007405
Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document
Abstract
Myocarditis is an inflammatory disease of the heart that may occur because of infections, immune system activation, or exposure to drugs. The diagnosis of myocarditis has changed due to the introduction of cardiac magnetic resonance imaging. We present an expert consensus document aimed to summarize the common terminology related to myocarditis meanwhile highlighting some areas of controversies and uncertainties and the unmet clinical needs. In fact, controversies persist regarding mechanisms that determine the transition from the initial trigger to myocardial inflammation and from acute myocardial damage to chronic ventricular dysfunction. It is still uncertain which viruses (besides enteroviruses) cause direct tissue damage, act as triggers for immune-mediated damage, or both. Regarding terminology, myocarditis can be characterized according to etiology, phase, and severity of the disease, predominant symptoms, and pathological findings. Clinically, acute myocarditis (AM) implies a short time elapsed from the onset of symptoms and diagnosis (generally <1 month). In contrast, chronic inflammatory cardiomyopathy indicates myocardial inflammation with established dilated cardiomyopathy or hypokinetic nondilated phenotype, which in the advanced stages evolves into fibrosis without detectable inflammation. Suggested diagnostic and treatment recommendations for AM and chronic inflammatory cardiomyopathy are mainly based on expert opinion given the lack of well-designed contemporary clinical studies in the field. We will provide a shared and practical approach to patient diagnosis and management, underlying differences between the European and US scientific statements on this topic. We explain the role of histology that defines subtypes of myocarditis and its prognostic and therapeutic implications.
Keywords: cardiac magnetic resonance imaging; endomyocardial biopsy; inflammatory cardiomyopathy; myocarditis; viruses.
Conflict of interest statement
Dr Adler is a consultant for Abbott, Abiomed, AstraZeneca, Endotronix, Ionis, Medtronic, and Novartis, is on the board of directors of Genstem Therapeutics, and is a shareholder of Rocket Pharmaceuticals. Dr Brambatti is an employee at Ionis Pharmaceuticals. Dr Moslehi has served on advisory boards for Pfizer, Novartis, Bristol-Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca, GlaxoSmithKline, Intrexon, and Regeneron and has been supported by National Institutes of Health grants R56 HL141466 and R01 HL141466. Dr Tschöpe is a consultant at Cardiotropic Labs, Miami, FL. Dr Camici is a consultant at Servier. The other authors report no conflicts.
Figures
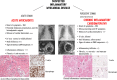

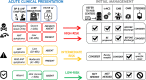
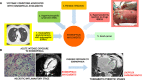
Comment in
-
Outcomes With Peripheral Venoarterial Extracorporeal Membrane Oxygenation for Suspected Acute Myocarditis: 10-Year Experience From the Extracorporeal Life Support Organization Registry.Circ Heart Fail. 2023 Jul;16(7):e010152. doi: 10.1161/CIRCHEARTFAILURE.122.010152. Epub 2023 Jun 22. Circ Heart Fail. 2023. PMID: 37345545
References
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013;34:2636–48, 2648a. doi: 10.1093/eurheartj/eht210 - PubMed
-
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841 - PubMed
-
- Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1 - PubMed
-
- Harmon KG, Drezner JA, Maleszewski JJ, Lopez-Anderson M, Owens D, Prutkin JM, Asif IM, Klossner D, Ackerman MJ. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ Arrhythm Electrophysiol. 2014;7:198–204. doi: 10.1161/CIRCEP.113.001376 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

