Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy
- PMID: 33176598
- PMCID: PMC8529295
- DOI: 10.1177/0300891620974755
Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy
Abstract
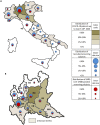
There are no robust data on the real onset of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and spread in the prepandemic period worldwide. We investigated the presence of SARS-CoV-2 receptor-binding domain (RBD)-specific antibodies in blood samples of 959 asymptomatic individuals enrolled in a prospective lung cancer screening trial between September 2019 and March 2020 to track the date of onset, frequency, and temporal and geographic variations across the Italian regions. SARS-CoV-2 RBD-specific antibodies were detected in 111 of 959 (11.6%) individuals, starting from September 2019 (14%), with a cluster of positive cases (>30%) in the second week of February 2020 and the highest number (53.2%) in Lombardy. This study shows an unexpected very early circulation of SARS-CoV-2 among asymptomatic individuals in Italy several months before the first patient was identified, and clarifies the onset and spread of the coronavirus disease 2019 (COVID-19) pandemic. Finding SARS-CoV-2 antibodies in asymptomatic people before the COVID-19 outbreak in Italy may reshape the history of pandemic.
Keywords: COVID-19; SARS-CoV-2 antibodies; Screening.
Conflict of interest statement
Figures

Comment in
-
Unexpected volume of Google searches for COVID-19 symptoms in the prepandemic period in Lombardia, Italy.Tumori. 2021 Oct;107(5):468-469. doi: 10.1177/0300891620980796. Epub 2020 Dec 16. Tumori. 2021. PMID: 33327872 No abstract available.
-
Comments on: "Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy".Tumori. 2021 Oct;107(5):470-471. doi: 10.1177/0300891621992430. Epub 2021 Feb 8. Tumori. 2021. PMID: 33557715 No abstract available.
-
Publisher's Note: Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy.Tumori. 2021 Oct;107(5):474. doi: 10.1177/0300891620987756. Epub 2021 Mar 22. Tumori. 2021. PMID: 33752533 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

