The piperazine compound ASP activates an auxin response in Arabidopsis thaliana
- PMID: 33176686
- PMCID: PMC7659159
- DOI: 10.1186/s12864-020-07203-8
The piperazine compound ASP activates an auxin response in Arabidopsis thaliana
Abstract
Background: Auxins play key roles in the phytohormone network. Early auxin response genes in the AUX/IAA, SAUR, and GH3 families show functional redundancy, which makes it very difficult to study the functions of individual genes based on gene knockout analysis or transgenic technology. As an alternative, chemical genetics provides a powerful approach that can be used to address questions relating to plant hormones.
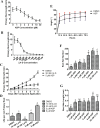
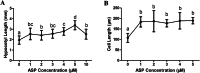
Results: By screening a small-molecule chemical library of compounds that can induce abnormal seedling and vein development, we identified and characterized a piperazine compound 1-[(4-bromophenoxy) acetyl]-4-[(4-fluorophenyl) sulfonyl] piperazine (ASP). The Arabidopsis DR5::GFP line was used to assess if the effects mentioned were correlated with the auxin response, and we accordingly verified that ASP altered the auxin-related pathway. Subsequently, we examined the regulatory roles of ASP in hypocotyl and root development, auxin distribution, and changes in gene expression. Following ASP treatment, we detected hypocotyl elongation concomitant with enhanced cell elongation. Furthermore, seedlings showed retarded primary root growth, reduced gravitropism and increased root hair development. These phenotypes were associated with an increased induction of DR5::GUS expression in the root/stem transition zone and root tips. Auxin-related mutants including tir1-1, aux1-7 and axr2-1 showed phenotypes with different root-development pattern from that of the wild type (Col-0), and were insensitive to ASP. Confocal images of propidium iodide (PI)-stained root tip cells showed no detectable damage by ASP. Furthermore, RT-qPCR analyses of two other genes, namely, Ethylene Response Factor (ERF115) and Mediator 18 (MED18), which are related to cell regeneration and damage, indicated that the ASP inhibitory effect on root growth was not attributable to toxicity. RT-qPCR analysis provided further evidence that ASP induced the expression of early auxin-response-related genes.
Conclusions: ASP altered the auxin response pathway and regulated Arabidopsis growth and development. These results provide a basis for dissecting specific molecular components involved in auxin-regulated developmental processes and offer new opportunities to discover novel molecular players involved in the auxin response.
Keywords: ASP; Auxin response; Auxin signaling; Chemical genetics; Phytohormone.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

