Treating male partners of women with bacterial vaginosis (StepUp): a protocol for a randomised controlled trial to assess the clinical effectiveness of male partner treatment for reducing the risk of BV recurrence
- PMID: 33176727
- PMCID: PMC7661182
- DOI: 10.1186/s12879-020-05563-w
Treating male partners of women with bacterial vaginosis (StepUp): a protocol for a randomised controlled trial to assess the clinical effectiveness of male partner treatment for reducing the risk of BV recurrence
Abstract
Background: Bacterial vaginosis (BV) is estimated to affect 1 in 3 women globally and is associated with obstetric and gynaecological sequelae. Current recommended therapies have good short-term efficacy but 1 in 2 women experience BV recurrence within 6 months of treatment. Evidence of male carriage of BV-organisms suggests that male partners may be reinfecting women with BV-associated bacteria (henceforth referred to as BV-organisms) and impacting on the efficacy of treatment approaches solely directed to women. This trial aims to determine the effect of concurrent male partner treatment for preventing BV recurrence compared to current standard of care.
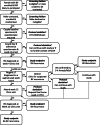
Methods: StepUp is an open-label, multicentre, parallel group randomised controlled trial for women diagnosed with BV and their male partner. Women with clinical-BV defined using current gold standard diagnosis methods (≥3 Amsel criteria and Nugent score (NS) = 4-10) and with a regular male partner will be assessed for eligibility, and couples will then be consented. All women will be prescribed oral metronidazole 400 mg twice daily (BID) for 7 days, or if contraindicated, a 7-day regimen of topical vaginal 2% clindamycin. Couples will be randomised 1:1 to either current standard of care (female treatment only), or female treatment and concurrent male partner treatment (7 days of combined antibiotics - oral metronidazole tablets 400 mg BID and 2% clindamycin cream applied topically to the glans penis and upper shaft [under the foreskin if uncircumcised] BID). Couples will be followed for up to 12 weeks to assess BV status in women, and assess the adherence, tolerability and acceptability of male partner treatment. The primary outcome is BV recurrence defined as ≥3 Amsel criteria and NS = 4-10 within 12 weeks of enrolment. The estimated sample size is 342 couples, to detect a 40% reduction in BV recurrence rates from 40% in the control group to 24% in the intervention group within 12 weeks.
Discussion: Current treatments directed solely to women result in unacceptably high rates of BV recurrence. If proven to be effective the findings from this trial will directly inform the development of new treatment strategies to impact on BV recurrence.
Trial registration: The trial was prospectively registered on 12 February 2019 on the Australian and New Zealand Clinical Trial Registry (ACTRN12619000196145, Universal Trial Number: U1111-1228-0106, https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=376883&isReview=true ).
Keywords: Antibiotic treatment; Bacterial vaginosis; Clindamycin; Couple treatment; Metronidazole; Recurrence; StepUp.
Conflict of interest statement
Not applicable.
Figures

References
-
- Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis. 2019;46(5):304–311. - PubMed
-
- Koumans EH, Markowitz LE, Berman SM, St Louis ME. A public health approach to adverse outcomes of pregnancy associated with bacterial vaginosis. Int J Gynaecol Obstet. 1999;67:S29–S33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

