In vivo RyR1 reduction in muscle triggers a core-like myopathy
- PMID: 33176865
- PMCID: PMC7657350
- DOI: 10.1186/s40478-020-01068-4
In vivo RyR1 reduction in muscle triggers a core-like myopathy
Abstract
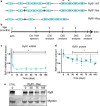
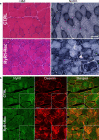
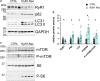
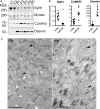
Mutations in the RYR1 gene, encoding the skeletal muscle calcium channel RyR1, lead to congenital myopathies, through expression of a channel with abnormal permeability and/or in reduced amount, but the direct functional whole organism consequences of exclusive reduction in RyR1 amount have never been studied. We have developed and characterized a mouse model with inducible muscle specific RYR1 deletion. Tamoxifen-induced recombination in the RYR1 gene at adult age resulted in a progressive reduction in the protein amount reaching a stable level of 50% of the initial amount, and was associated with a progressive muscle weakness and atrophy. Measurement of calcium fluxes in isolated muscle fibers demonstrated a reduction in the amplitude of RyR1-related calcium release mirroring the reduction in the protein amount. Alterations in the muscle structure were observed, with fibers atrophy, abnormal mitochondria distribution and membrane remodeling. An increase in the expression level of many proteins was observed, as well as an inhibition of the autophagy process. This model demonstrates that RyR1 reduction is sufficient to recapitulate most features of Central Core Disease, and accordingly similar alterations were observed in muscle biopsies from Dusty Core Disease patients (a subtype of Central Core Disease), pointing to common pathophysiological mechanisms related to RyR1 reduction.
Keywords: Calcium; Central core disease; Congenital myopathies; Dusty core disease; Excitation–contraction coupling; Mouse model; Ryanodine receptor; Skeletal muscle.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

