Linking Social Cognition to Learning and Memory
- PMID: 33177112
- PMCID: PMC7659449
- DOI: 10.1523/JNEUROSCI.1280-20.2020
Linking Social Cognition to Learning and Memory
Abstract
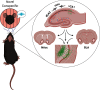
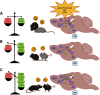
Many mammals have evolved to be social creatures. In humans, the ability to learn from others' experiences is essential to survival; and from an early age, individuals are surrounded by a social environment that helps them develop a variety of skills, such as walking, talking, and avoiding danger. Similarly, in rodents, behaviors, such as food preference, exploration of novel contexts, and social approach, can be learned through social interaction. Social encounters facilitate new learning and help modify preexisting memories throughout the lifespan of an organism. Moreover, social encounters can help buffer stress or the effects of negative memories, as well as extinguish maladaptive behaviors. Given the importance of such interactions, there has been increasing work studying social learning and applying its concepts in a wide range of fields, including psychotherapy and medical sociology. The process of social learning, including its neural and behavioral mechanisms, has also been a rapidly growing field of interest in neuroscience. However, the term "social learning" has been loosely applied to a variety of psychological phenomena, often without clear definition or delineations. Therefore, this review gives a definition for specific aspects of social learning, provides an overview of previous work at the circuit, systems, and behavioral levels, and finally, introduces new findings on the social modulation of learning. We contextualize such social processes in the brain both through the role of the hippocampus and its capacity to process "social engrams" as well as through the brainwide realization of social experiences. With the integration of new technologies, such as optogenetics, chemogenetics, and calcium imaging, manipulating social engrams will likely offer a novel therapeutic target to enhance the positive buffering effects of social experiences or to inhibit fear-inducing social stimuli in models of anxiety and post-traumatic stress disorder.
Keywords: behavior; engram; learning; memory; optogenetics; social.
Copyright © 2020 The authors.
Figures



References
-
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, Cum MI, Stergiadou J, Anandalingam KK, Farris K, Namburi P, Leppla CA, Weddington JC, Nieh EH, Smith AC, Ba D, et al. (2018) Corticoamygdala transfer of socially derived information gates observational learning. Cell 173:1329–1342.e18. 10.1016/j.cell.2018.04.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
