A double-blind, randomized, placebo- and positive-controlled phase III trial of 1% benvitimod cream in mild-to-moderate plaque psoriasis
- PMID: 33177393
- PMCID: PMC7752683
- DOI: 10.1097/CM9.0000000000001221
A double-blind, randomized, placebo- and positive-controlled phase III trial of 1% benvitimod cream in mild-to-moderate plaque psoriasis
Abstract
Background: Benvitimod cream, a novel synthetic small molecule, was effective in treating mild-to-moderate plaque psoriasis. We conducted a phase III clinical trial to assess the efficacy and safety of benvitimod cream in patients with mild-to-moderate plaque psoriasis.
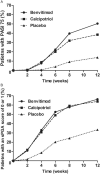
Methods: We randomly assigned 686 patients (2:1:1) to receive 1% benvitimod cream, 0.005% calcipotriol ointment or placebo twice a day for 12 weeks. The primary efficacy end points were the percentage of patients with a 75% or greater reduction from baseline in the psoriasis area and severity index (PASI 75) score and with a score of 0 or 1 in static physician's global assessment (sPGA) at week 12.
Results: The results showed that 50.4% of patients in the benvitimod group achieved PASI 75, which was significantly higher than that in the calcipotriol (38.5%, P < 0.05) and placebo (13.9%, P < 0.05) groups. The proportion of patients achieving an sPGA score 0 or 1 was 66.3% in the benvitimod group and 63.9% in the calcipotriol group, which were both significantly higher than that in the placebo group (34%, P < 0.05). In the long-term follow-up study, 50.8% of patients experienced recurrence. After retreatment with 1% benvitimod, 73.3% of patients achieved an sPGA score of 0 or 1 again at week 52. Adverse events included application site irritation, follicular papules, and contact dermatitis. No systemic adverse reactions were reported.
Conclusion: During this 12-week study, benvitimod cream was demonstrated with high effectiveness and safety in patients with mild-to-moderate plaque psoriasis.
Trial registration: Chinese Clinical Trial Registry (ChiCTR), ChiCTR-TRC-13003259; http://www.chictr.org.cn/showprojen.aspx?proj=6300.
Copyright © 2020 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures
References
-
- Mounsey SJ, Elizabeth K. Psoriasis. Br J Hosp Med 2018; 79:C114–C117. doi: 10.12968/hmed.2018.79.8.C114. - PubMed
-
- Hugh JM, Weinberg JM. Update on the pathophysiology of psoriasis. Cutis 2018; 102:6–12. - PubMed
-
- Michalek I, Loring B, John S. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31:205–212. doi: 10.1111/jdv.13854. - PubMed
-
- Gabros S, Nessel TA, Zito PM. Topical Corticosteroids. [Updated 2020 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532940/. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

