Structural organization of the bone marrow and its role in hematopoiesis
- PMID: 33177411
- PMCID: PMC7769132
- DOI: 10.1097/MOH.0000000000000621
Structural organization of the bone marrow and its role in hematopoiesis
Abstract
Purpose of review: The bone marrow is the main site for hematopoiesis. It contains a unique microenvironment that provides niches that support self-renewal and differentiation of hematopoietic stem cells (HSC), multipotent progenitors (MPP), and lineage committed progenitors to produce the large number of blood cells required to sustain life. The bone marrow is notoriously difficult to image; because of this the anatomy of blood cell production -- and how local signals spatially organize hematopoiesis -- are not well defined. Here we review our current understanding of the spatial organization of the mouse bone marrow with a special focus in recent advances that are transforming our understanding of this tissue.
Recent findings: Imaging studies of HSC and their interaction with candidate niches have relied on ex-vivo imaging of fixed tissue. Two recent manuscripts demonstrating live imaging of subsets of HSC in unperturbed bone marrow have revealed unexpected HSC behavior and open the door to examine HSC regulation, in situ, over time. We also discuss recent findings showing that the bone marrow contains distinct microenvironments, spatially organized, that regulate unique aspects of hematopoiesis.
Summary: Defining the spatial architecture of hematopoiesis in the bone marrow is indispensable to understand how this tissue ensures stepwise, balanced, differentiation to meet organism demand; for deciphering alterations to hematopoiesis during disease; and for designing organ systems for blood cell production ex vivo.
Conflict of interest statement
Conflicts of interest
The author has no conflicts of interest
Figures
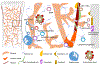
References
-
- Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR: Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 2015, 518:542–546. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

