Dengue virus infection impedes megakaryopoiesis in MEG-01 cells where the virus envelope protein interacts with the transcription factor TAL-1
- PMID: 33177556
- PMCID: PMC7658202
- DOI: 10.1038/s41598-020-76350-5
Dengue virus infection impedes megakaryopoiesis in MEG-01 cells where the virus envelope protein interacts with the transcription factor TAL-1
Abstract
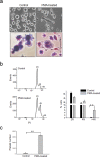
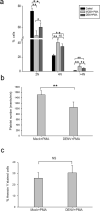
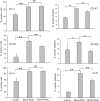
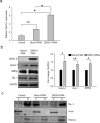
Dengue virus (DENV) infection causes dengue fever in humans, which can lead to thrombocytopenia showing a marked reduction in platelet counts, and dengue hemorrhagic fever. The virus may cause thrombocytopenia either by destroying the platelets or by interfering with their generation via the process of megakaryopoiesis. MEG-01 is the human megakaryoblastic leukemia cell line that can be differentiated in vitro by phorbol-12-myristate-13-acetate (PMA) treatment to produce platelet-like-particles (PLPs). We have studied DENV infection of MEG-01 cells to understand its effect on megakaryopoiesis and the generation of PLPs. We observed that DENV could infect only naive MEG-01 cells, and differentiated cells were refractory to virus infection/replication. However, DENV-infected MEG-01 cells, when induced for differentiation with PMA, supported an enhanced viral replication. Following the virus infection, the MEG-01 cells showed a marked reduction in the surface expression of platelet markers (CD41, CD42a, and CD61), a decreased polyploidy, and significantly reduced PLP counts. DENV infection caused an enhanced Notch signaling in MEG-01 cells where the virus envelope protein was shown to interact with TAL-1, a host protein important for megakaryopoiesis. These observations provide new insight into the role of DENV in modulating the megakaryopoiesis and platelet production process.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Word Health Organization . Dengue: Guidelines for Diagnosis, Treatment. Geneva: Prevention and Control. WHO; 2009. - PubMed
-
- de Azeredo EL, et al. Mediat. Inflamm. Mediat. Inflamm. 2015;2015:e313842.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

