Electroporation outperforms in vivo-jetPEI for intratumoral DNA-based reporter gene transfer
- PMID: 33177564
- PMCID: PMC7659317
- DOI: 10.1038/s41598-020-75206-2
Electroporation outperforms in vivo-jetPEI for intratumoral DNA-based reporter gene transfer
Abstract
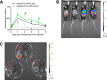
Intratumoral delivery of drug-encoding plasmid DNA (pDNA) enables localised in vivo expression of biological drugs, offering an attractive alternative to conventional protein treatment. However, this requires physical or chemical methods to enhance the low transfection efficiency of naked pDNA. Electroporation and complexation with the polycation in vivo-jetPEI are both evaluated in the clinic for intratumoral pDNA delivery, but lack head-to-head comparison. This study therefore compared both methods for intratumoral DNA-based reporter gene transfer in a subcutaneous mouse tumour model. Intratumoral electroporation resulted in strong reporter expression that was restricted to the tumour area and persisted for at least ten days. Intratumoral expression after injection of pDNA-jetPEI complexes was two to three logs lower, did not exceed the background in most mice, and lasted less than five days even with repeated dosing. Remarkably, reporter expression was primarily detected in the lungs, presumably due to leakage of pDNA-jetPEI complexes into the systemic circulation. In conclusion, electroporation enabled more efficient, prolonged and tumour-specific reporter expression compared to intratumoral injection of pDNA complexed with in vivo-jetPEI. These results favour the use of electroporation for intratumoral DNA-based gene transfer, and suggest further optimisation of pDNA-jetPEI complexes is needed to improve their efficacy and biosafety.
Conflict of interest statement
K.H. received consulting fees from OncoSec Medical (San Diego, CA, USA) in 2018. All other authors declare no conflicts of interest.
Figures

References
-
- Annan AC, Fisher PB, Dent P, Siegal GP, Curiel DT. Gene Therapy in the Treatment of Human Cancer. In: Coleman W, Tsongalis G, editors. The Molecular Basis of Human Cancer. Totowa: Humana Press; 2017. pp. 811–841.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

