The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes
- PMID: 33177612
- PMCID: PMC7659000
- DOI: 10.1038/s41598-020-76505-4
The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes
Abstract
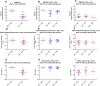
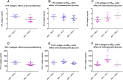
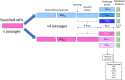
Cardiovascular disease is the primary driver of morbidity and mortality associated with diabetes. Hyperglycaemia is implicated in driving endothelial dysfunction that might underpin the link between diabetes and cardiovascular disease. This study was designed to determine the impact of chronic preconditioning of cells to hyperglycaemia and transient switching of cultured endothelial cells between hyper- and normo-glycaemic conditions on bioenergetic and functional parameters. Immortalised EA.hy926 endothelial cells were cultured through multiple passages under normoglycaemic (5.5 mM) or hyperglycaemic (25 mM) conditions. Cells were subsequently subjected (48 h) to continued normo- or hyperglycaemic exposure, or were switched to the alternative glycaemic condition, or to an intermediate glucose concentration (12.5 mM) and metabolic activity, together with key markers of function were measured. Cells habituated to hyperglycaemia were energetically quiescent. Functional activity, characterised by the measurement of nitric oxide, endothelin-1, tissue plasminogen activator and plasminogen activator inhibitor-1, was depressed by exposure to high glucose, with the reduction in nitric oxide production being the most notable. Function was more responsive to acute changes in extracellular glucose than were bioenergetic changes. We conclude that glucose is a key determinant of endothelial function. The study highlights the importance of chronic glucose exposure on cell phenotype and emphasises the need to pay close attention to glucose preconditioning in interpreting results under culture conditions.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ceriello A, Ihnat MA, Thorpe JE. The ‘Metabolic memory’: Is more than just tight glucose control necessary to prevent diabetic complications? J. Clin. Endocrinol. Metab. 2009;94:410–415. - PubMed
-
- Misra A, Bloomgarden Z. Metabolic memory: Evolving concepts. J. Diabetes. 2018;10:186–187. - PubMed
-
- Drzewoski J, Kasznicki J, Trojanowski Z. The role of ‘metabolic memory’ in the natural history of diabetes mellitus. Pol. Arch. Med. Wewn. 2009;119:493–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

