Predictive modeling of proliferative vitreoretinopathy using automated machine learning by ophthalmologists without coding experience
- PMID: 33177614
- PMCID: PMC7658348
- DOI: 10.1038/s41598-020-76665-3
Predictive modeling of proliferative vitreoretinopathy using automated machine learning by ophthalmologists without coding experience
Abstract
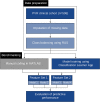
We aimed to assess the feasibility of machine learning (ML) algorithm design to predict proliferative vitreoretinopathy (PVR) by ophthalmologists without coding experience using automated ML (AutoML). The study was a retrospective cohort study of 506 eyes who underwent pars plana vitrectomy for rhegmatogenous retinal detachment (RRD) by a single surgeon at a tertiary-care hospital between 2012 and 2019. Two ophthalmologists without coding experience used an interactive application in MATLAB to build and evaluate ML algorithms for the prediction of postoperative PVR using clinical data from the electronic health records. The clinical features associated with postoperative PVR were determined by univariate feature selection. The area under the curve (AUC) for predicting postoperative PVR was better for models that included pre-existing PVR as an input. The quadratic support vector machine (SVM) model built using all selected clinical features had an AUC of 0.90, a sensitivity of 63.0%, and a specificity of 97.8%. An optimized Naïve Bayes algorithm that did not include pre-existing PVR as an input feature had an AUC of 0.81, a sensitivity of 54.3%, and a specificity of 92.4%. In conclusion, the development of ML models for the prediction of PVR by ophthalmologists without coding experience is feasible. Input from a data scientist might still be needed to tackle class imbalance-a common challenge in ML classification using real-world clinical data.
Conflict of interest statement
The authors declare no competing interests
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

