Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation
- PMID: 33177619
- PMCID: PMC8027700
- DOI: 10.1038/s41418-020-00652-4
Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation
Abstract
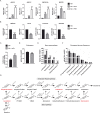
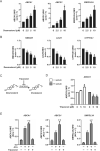
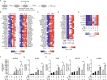
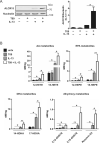
Macrophages acquire anti-inflammatory and proresolving functions to facilitate resolution of inflammation and promote tissue repair. While alternatively activated macrophages (AAMs), also referred to as M2 macrophages, polarized by type 2 (Th2) cytokines IL-4 or IL-13 contribute to the suppression of inflammatory responses and play a pivotal role in wound healing, contemporaneous exposure to apoptotic cells (ACs) potentiates the expression of anti-inflammatory and tissue repair genes. Given that liver X receptors (LXRs), which coordinate sterol metabolism and immune cell function, play an essential role in the clearance of ACs, we investigated whether LXR activation following engulfment of ACs selectively potentiates the expression of Th2 cytokine-dependent genes in primary human AAMs. We show that AC uptake simultaneously upregulates LXR-dependent, but suppresses SREBP-2-dependent gene expression in macrophages, which are both prevented by inhibiting Niemann-Pick C1 (NPC1)-mediated sterol transport from lysosomes. Concurrently, macrophages accumulate sterol biosynthetic intermediates desmosterol, lathosterol, lanosterol, and dihydrolanosterol but not cholesterol-derived oxysterols. Using global transcriptome analysis, we identify anti-inflammatory and proresolving genes including interleukin-1 receptor antagonist (IL1RN) and arachidonate 15-lipoxygenase (ALOX15) whose expression are selectively potentiated in macrophages upon concomitant exposure to ACs or LXR agonist T0901317 (T09) and Th2 cytokines. We show priming macrophages via LXR activation enhances the cellular capacity to synthesize inflammation-suppressing specialized proresolving mediator (SPM) precursors 15-HETE and 17-HDHA as well as resolvin D5. Silencing LXRα and LXRβ in macrophages attenuates the potentiation of ALOX15 expression by concomitant stimulation of ACs or T09 and IL-13. Collectively, we identify a previously unrecognized mechanism of regulation whereby LXR integrates AC uptake to selectively shape Th2-dependent gene expression in AAMs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. - PubMed
-
- Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121:e57–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

