The role of soluble mediators in the clinical course of EBV infection and B cell homeostasis after kidney transplantation
- PMID: 33177622
- PMCID: PMC7658229
- DOI: 10.1038/s41598-020-76607-z
The role of soluble mediators in the clinical course of EBV infection and B cell homeostasis after kidney transplantation
Abstract
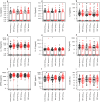
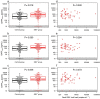
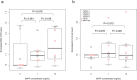
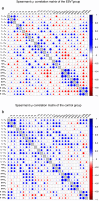
Epstein-Barr virus (EBV) reactivation can lead to serious complications in kidney transplant patients, including post-transplant lymphoproliferative disorder (PTLD). Here, we have assessed the impact of EBV on B cell homeostasis at cellular and humoral level. In a multicenter study monitoring 540 kidney transplant patients during the first post-transplant year, EBV reactivation was detected in 109 patients. Thirteen soluble factors and B cell counts were analyzed in an EBV+ sub-cohort (N = 54) before, at peak and after EBV clearance and compared to a control group (N = 50). The B cell activating factor (BAFF) was significantly elevated among EBV+ patients. No additional soluble factors were associated with EBV. Importantly, in vitro experiments confirmed the proliferative effect of BAFF on EBV-infected B cells, simultaneously promoting EBV production. In contrast, elevated levels of BAFF in EBV+ patients did not lead to B cell expansion in vivo. Moreover, diminished positive inter-correlations of soluble factors and alterations of the bi-directional interplay between B cell and soluble factors were observed in EBV+ patients at peak and after clearance. Our data suggest that such alterations may counteract the proliferative effect of BAFF, preventing B cell expansion. The role of these alterations in lymphoma development should be analyzed in future studies.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N. Engl. J. Med. 2018;378:549–562. - PubMed
-
- Petrara MR, et al. Post-transplant lymphoproliferative disorders: From epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37–44. - PubMed
-
- Andrei, G., Trompet, E. & Snoeck, R. Novel therapeutics for Epstein–Barr virus. 10.3390/molecules24050997 (2019).
-
- Ng S-B, Khoury JD. Epstein-Barr virus in lymphoproliferative processes: An update for the diagnostic pathologist. Adv. Anat. Pathol. 2009;16:40–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

