EZH2 inhibition reduces cartilage loss and functional impairment related to osteoarthritis
- PMID: 33177650
- PMCID: PMC7658239
- DOI: 10.1038/s41598-020-76724-9
EZH2 inhibition reduces cartilage loss and functional impairment related to osteoarthritis
Abstract
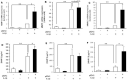
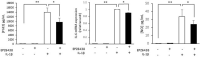
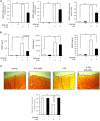
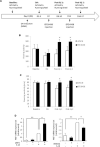
Histone methyltransferase EZH2 is upregulated during osteoarthritis (OA), which is the most widespread rheumatic disease worldwide, and a leading cause of disability. This study aimed to assess the impact of EZH2 inhibition on cartilage degradation, inflammation and functional disability. In vitro, gain and loss of EZH2 function were performed in human articular OA chondrocytes stimulated with IL-1β. In vivo, the effects of EZH2 inhibition were investigated on medial meniscectomy (MMX) OA mouse model. The tissue alterations were assayed by histology and the functional disabilities of the mice by actimetry and running wheel. In vitro, EZH2 overexpression exacerbated the action of IL-1β in chondrocytes increasing the expression of genes involved in inflammation, pain (NO, PGE2, IL6, NGF) and catabolism (MMPs), whereas EZH2 inhibition by a pharmacological inhibitor, EPZ-6438, reduced IL-1β effects. Ex vivo, EZH2 inhibition decreased IL-1β-induced degradation of cartilage. In vivo, intra-articular injections of the EZH2 inhibitor reduced cartilage degradation and improved motor functions of OA mice. This study demonstrates that the pharmacological inhibition of the histone methyl-transferase EZH2 slows the progression of osteoarthritis and improves motor functions in an experimental OA model, suggesting that EZH2 could be an effective target for the treatment of OA by reducing catabolism, inflammation and pain.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435. doi: 10.1136/bmjopen-2011-000435. - DOI - PMC - PubMed
-
- Hawker G. Osteoarthritis is a serious disease. Clin Exp Rheumatol. 2019;37:3–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

