Antenatal corticosteroids: a reappraisal of the drug formulation and dose
- PMID: 33177675
- PMCID: PMC7892336
- DOI: 10.1038/s41390-020-01249-w
Antenatal corticosteroids: a reappraisal of the drug formulation and dose
Abstract
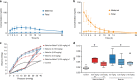
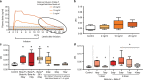
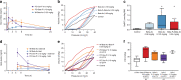
We review the history of antenatal corticosteroid therapy (ACS) and present recent experimental data to demonstrate that this, one of the pillars of perinatal care, has been inadequately evaluated to minimize fetal exposure to these powerful medications. There have been concerns since 1972 that fetal exposures to ACS convey risk. However, this developmental modulator, with its multiple widespread biologic effects, has not been evaluated for drug choice, dose, or duration of treatment, despite over 30 randomized trials. The treatment used in the United States is two intramuscular doses of a mixture of 6 mg betamethasone phosphate (Beta P) and 6 mg betamethasone acetate (Beta Ac). To optimize outcomes with ACS, the goal should be to minimize fetal drug exposure. We have determined that the minimum exposure needed for fetal lung maturation in sheep, monkeys, and humans (based on published cord blood corticosteroid concentrations) is about 1 ng/ml for a 48-h continuous exposure, far lower than the concentration reached by the current dosing. Because the slowly released Beta Ac results in prolonged fetal exposure, a drug containing Beta Ac is not ideal for ACS use. IMPACT: Using sheep and monkey models, we have defined the minimum corticosteroid exposure for a fetal lung maturation. These results should generate new clinical trials of antenatal corticosteroids (ACS) at much lower fetal exposures to ACS, possibly given orally, with fewer risks for the fetus.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Committee Opinion No. 713 summary: antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 2017;130:493–494. - PubMed
-
- WHO Recommendations on Interventions to Improve Preterm Birth Outcomes (WHO Guidelines Approved by the Guidelines Review Committee, Geneva, 2015). - PubMed
-
- World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. - PubMed
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. - PubMed
-
- Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J. Endocrinol. 1969;45:515–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

