Chemico-genetic discovery of astrocytic control of inhibition in vivo
- PMID: 33177716
- PMCID: PMC8011649
- DOI: 10.1038/s41586-020-2926-0
Chemico-genetic discovery of astrocytic control of inhibition in vivo
Abstract
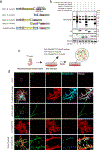
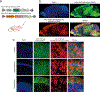
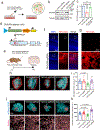
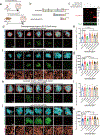
Perisynaptic astrocytic processes are an integral part of central nervous system synapses1,2; however, the molecular mechanisms that govern astrocyte-synapse adhesions and how astrocyte contacts control synapse formation and function are largely unknown. Here we use an in vivo chemico-genetic approach that applies a cell-surface fragment complementation strategy, Split-TurboID, and identify a proteome that is enriched at astrocyte-neuron junctions in vivo, which includes neuronal cell adhesion molecule (NRCAM). We find that NRCAM is expressed in cortical astrocytes, localizes to perisynaptic contacts and is required to restrict neuropil infiltration by astrocytic processes. Furthermore, we show that astrocytic NRCAM interacts transcellularly with neuronal NRCAM coupled to gephyrin at inhibitory postsynapses. Depletion of astrocytic NRCAM reduces numbers of inhibitory synapses without altering glutamatergic synaptic density. Moreover, loss of astrocytic NRCAM markedly decreases inhibitory synaptic function, with minor effects on excitation. Thus, our results present a proteomic framework for how astrocytes interface with neurons and reveal how astrocytes control GABAergic synapse formation and function.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

