Hippocampal Hyperactivity as a Druggable Circuit-Level Origin of Aberrant Salience in Schizophrenia
- PMID: 33178010
- PMCID: PMC7596262
- DOI: 10.3389/fphar.2020.486811
Hippocampal Hyperactivity as a Druggable Circuit-Level Origin of Aberrant Salience in Schizophrenia
Abstract
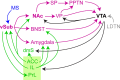
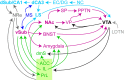
The development of current neuroleptics was largely aiming to decrease excessive dopaminergic signaling in the striatum. However, the notion that abnormal dopamine creates psychotic symptoms by causing an aberrant assignment of salience that drives maladaptive learning chronically during disease development suggests a therapeutic value of early interventions that correct salience-related neural processing. The mesolimbic dopaminergic output is modulated by several interconnected brain-wide circuits centrally involving the hippocampus and key relays like the ventral and associative striatum, ventral pallidum, amygdala, bed nucleus of the stria terminalis, nucleus reuniens, lateral and medial septum, prefrontal and cingulate cortex, among others. Unraveling the causal relationships between these circuits using modern neuroscience techniques holds promise for identifying novel cellular-and ultimately molecular-treatment targets for reducing transition to psychosis and symptoms of schizophrenia. Imaging studies in humans have implicated a hyperactivity of the hippocampus as a robust and early endophenotype in schizophrenia. Experiments in rodents, in turn, suggested that the activity of its output region-the ventral subiculum-may modulate dopamine release from ventral tegmental area (VTA) neurons in the ventral striatum. Even though these observations suggested a novel circuit-level target for anti-psychotic action, no therapy has yet been developed along this rationale. Recently evaluated treatment strategies-at least in part-target excess glutamatergic activity, e.g. N-acetyl-cysteine (NAC), levetiracetam, and mGluR2/3 modulators. We here review the evidence for the central implication of the hippocampus-VTA axis in schizophrenia-related pathology, discuss its symptom-related implications with a particular focus on aberrant assignment of salience, and evaluate some of its short-comings and prospects for drug discovery.
Keywords: CA3; aberrant salience; attention; glutamate hypothesis; hippocampus; mesolimbic; schizophrenia; subiculum.
Copyright © 2020 Kätzel, Wolff, Bygrave and Bannerman.
Figures

References
-
- Adams D. H., Kinon B. J., Baygani S., Millen B. A., Velona I., Kollack-Walker S., et al. (2013). A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry 13:143. 10.1186/1471-244X-13-143 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

