Continuous, Automated Breathing Rate and Body Motion Monitoring of Rats With Paraquat-Induced Progressive Lung Injury
- PMID: 33178039
- PMCID: PMC7596732
- DOI: 10.3389/fphys.2020.569001
Continuous, Automated Breathing Rate and Body Motion Monitoring of Rats With Paraquat-Induced Progressive Lung Injury
Abstract
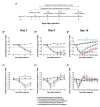
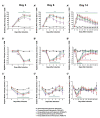
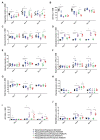
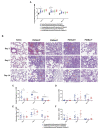
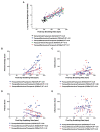
Assessments of respiratory response and animal activity are useful endpoints in drug pharmacology and safety research. We investigated whether continuous, direct monitoring of breathing rate and body motion in animals in the home cage using the Vum Digital Smart House can complement standard measurements in enabling more granular detection of the onset and severity of physiologic events related to lung injury in a well-established rodent model of paraquat (PQ) toxicity. In rats administered PQ, breathing rate was significantly elevated while body motion was significantly reduced following dosing and extending throughout the 14-day study duration for breathing rate and at least 5 days for both nighttime and daytime body motion. Time course differences in these endpoints in response to the potential ameliorative test article bardoxolone were also readily detected. More complete than standard in-life measurements, breathing rate and body motion tracked injury progression continuously over the full study time period and aligned with, and informed on interval changes in clinical pathology. In addition, breathing rates correlated with terminal pathology measurements, such as normalized lung weights and histologic alveolar damage and edema. This study is a preliminary evaluation of the technology; our results demonstrate that continuously measured breathing rate and body motion served as physiologically relevant readouts to assess lung injury progression and drug response in a respiratory injury animal model.
Keywords: activity; breathing rate; digital biomarkers; drug discovery; lung injury; paraquat; rodent; translational research.
Copyright © 2020 Baran, Gupta, Lim, Mathur, Rowlands, Schaevitz, Shanmukhappa and Walker.
Figures
References
LinkOut - more resources
Full Text Sources

