Remodeling of the Microvasculature: May the Blood Flow Be With You
- PMID: 33178049
- PMCID: PMC7593767
- DOI: 10.3389/fphys.2020.586852
Remodeling of the Microvasculature: May the Blood Flow Be With You
Abstract
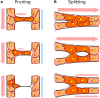
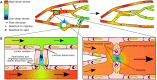
The vasculature ensures optimal delivery of nutrients and oxygen throughout the body, and to achieve this function it must continually adapt to varying tissue demands. Newly formed vascular plexuses during development are immature and require dynamic remodeling to generate well-patterned functional networks. This is achieved by remodeling of the capillaries preserving those which are functional and eliminating other ones. A balanced and dynamically regulated capillary remodeling will therefore ensure optimal distribution of blood and nutrients to the tissues. This is particularly important in pathological contexts in which deficient or excessive vascular remodeling may worsen tissue perfusion and hamper tissue repair. Blood flow is a major determinant of microvascular reshaping since capillaries are pruned when relatively less perfused and they split when exposed to high flow in order to shape the microvascular network for optimal tissue perfusion and oxygenation. The molecular machinery underlying blood flow sensing by endothelial cells is being deciphered, but much less is known about how this translates into endothelial cell responses as alignment, polarization and directed migration to drive capillary remodeling, particularly in vivo. Part of this knowledge is theoretical from computational models since blood flow hemodynamics are not easily recapitulated by in vitro or ex vivo approaches. Moreover, these events are difficult to visualize in vivo due to their infrequency and briefness. Studies had been limited to postnatal mouse retina and vascular beds in zebrafish but new tools as advanced microscopy and image analysis are strengthening our understanding of capillary remodeling. In this review we introduce the concept of remodeling of the microvasculature and its relevance in physiology and pathology. We summarize the current knowledge on the mechanisms contributing to capillary regression and to capillary splitting highlighting the key role of blood flow to orchestrate these processes. Finally, we comment the potential and possibilities that microfluidics offers to this field. Since capillary remodeling mechanisms are often reactivated in prevalent pathologies as cancer and cardiovascular disease, all this knowledge could be eventually used to improve the functionality of capillary networks in diseased tissues and promote their repair.
Keywords: 3D-confocal microscopy; blood flow; capillary pruning; capillary splitting; endothelial cells; microfluidics; microvascular remodeling; shear stress.
Copyright © 2020 Santamaría, González-Álvarez, Delgado, Esteban and Arroyo.
Figures