Relationships Among Circulating Levels of Hemostasis Inhibitors, Chemokines, Adhesion Molecules, and MRI Characteristics in Multiple Sclerosis
- PMID: 33178104
- PMCID: PMC7593335
- DOI: 10.3389/fneur.2020.553616
Relationships Among Circulating Levels of Hemostasis Inhibitors, Chemokines, Adhesion Molecules, and MRI Characteristics in Multiple Sclerosis
Abstract
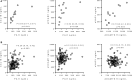
Background: Several studies suggested cross talk among components of hemostasis, inflammation, and immunity pathways in the pathogenesis, neurodegeneration, and occurrence of cerebral microbleeds (CMBs) in multiple sclerosis (MS). Objectives: This study aimed to evaluate the combined contribution of the hemostasis inhibitor protein C (PC) and chemokine C-C motif ligand 18 (CCL18) levels to brain atrophy in MS and to identify disease-relevant correlations among circulating levels of hemostasis inhibitors, chemokines, and adhesion molecules, particularly in CMB occurrence in MS. Methods: Plasma levels of hemostasis inhibitors (ADAMTS13, PC, and PAI1), CCL18, and soluble adhesion molecules (sNCAM, sICAM1, sVCAM1, and sVAP1) were evaluated by multiplex in 138 MS patients [85 relapsing-remitting (RR-MS) and 53 progressive (P-MS)] and 42 healthy individuals (HI) who underwent 3-T MRI exams. Association of protein levels with MRI outcomes was performed by regression analysis. Correlations among protein levels were assessed by partial correlation and Pearson's correlation. Results: In all patients, regression analysis showed that higher PC levels were associated with lower brain volumes, including the brain parenchyma (p = 0.002), gray matter (p < 0.001), cortex (p = 0.001), deep gray matter (p = 0.001), and thalamus (p = 0.001). These associations were detectable in RR-MS but not in P-MS patients. Higher CCL18 levels were associated with higher T2-lesion volumes in all MS patients (p = 0.03) and in the P-MS (p = 0.003). In the P-MS, higher CCL18 levels were also associated with lower volumes of the gray matter (p = 0.024), cortex (p = 0.043), deep gray matter (p = 0.029), and thalamus (p = 0.022). PC-CCL18 and CCL18-PAI1 levels were positively correlated in both MS and HI, PC-sVAP1 and PAI1-sVCAM1 only in MS, and PC-sICAM1 and PC-sNCAM only in HI. In MS patients with CMBs (n = 12), CCL18-PAI1 and PAI1-sVCAM1 levels were better correlated than those in MS patients without CMBs, and a novel ADAMTS13-sVAP1 level correlation (r = 0.78, p = 0.003) was observed. Conclusions: Differences between clinical phenotype groups in association of PC and CCL18 circulating levels with MRI outcomes might be related to different aspects of neurodegeneration. Disease-related pathway dysregulation is supported by several protein level correlation differences between MS patients and HI. The integrated analysis of plasma proteins and MRI measures provide evidence for new relationships among hemostasis, inflammation, and immunity pathways, relevant for MS and for the occurrence of CMBs.
Keywords: adhesion molecules; cerebral microbleeds; hemostasis inhibitors; multiple sclerosis; neurodegeneration.
Copyright © 2020 Ziliotto, Zivadinov, Jakimovski, Baroni, Bergsland, Ramasamy, Weinstock-Guttman, Ramanathan, Marchetti and Bernardi.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

