Neuroimaging Advances in Parkinson's Disease and Atypical Parkinsonian Syndromes
- PMID: 33178113
- PMCID: PMC7593544
- DOI: 10.3389/fneur.2020.572976
Neuroimaging Advances in Parkinson's Disease and Atypical Parkinsonian Syndromes
Abstract
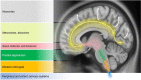
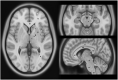
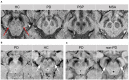
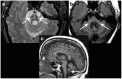
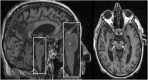
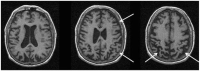
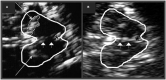
Parkinson's disease (PD) and atypical Parkinsonian syndromes are progressive heterogeneous neurodegenerative diseases that share clinical characteristic of parkinsonism as a common feature, but are considered distinct clinicopathological disorders. Based on the predominant protein aggregates observed within the brain, these disorders are categorized as, (1) α-synucleinopathies, which include PD and other Lewy body spectrum disorders as well as multiple system atrophy, and (2) tauopathies, which comprise progressive supranuclear palsy and corticobasal degeneration. Although, great strides have been made in neurodegenerative disease research since the first medical description of PD in 1817 by James Parkinson, these disorders remain a major diagnostic and treatment challenge. A valid diagnosis at early disease stages is of paramount importance, as it can help accommodate differential prognostic and disease management approaches, enable the elucidation of reliable clinicopathological relationships ideally at prodromal stages, as well as facilitate the evaluation of novel therapeutics in clinical trials. However, the pursuit for early diagnosis in PD and atypical Parkinsonian syndromes is hindered by substantial clinical and pathological heterogeneity, which can influence disease presentation and progression. Therefore, reliable neuroimaging biomarkers are required in order to enhance diagnostic certainty and ensure more informed diagnostic decisions. In this article, an updated presentation of well-established and emerging neuroimaging biomarkers are reviewed from the following modalities: (1) structural magnetic resonance imaging (MRI), (2) diffusion-weighted and diffusion tensor MRI, (3) resting-state and task-based functional MRI, (4) proton magnetic resonance spectroscopy, (5) transcranial B-mode sonography for measuring substantia nigra and lentiform nucleus echogenicity, (6) single photon emission computed tomography for assessing the dopaminergic system and cerebral perfusion, and (7) positron emission tomography for quantifying nigrostriatal functions, glucose metabolism, amyloid, tau and α-synuclein molecular imaging, as well as neuroinflammation. Multiple biomarkers obtained from different neuroimaging modalities can provide distinct yet corroborative information on the underlying neurodegenerative processes. This integrative "multimodal approach" may prove superior to single modality-based methods. Indeed, owing to the international, multi-centered, collaborative research initiatives as well as refinements in neuroimaging technology that are currently underway, the upcoming decades will mark a pivotal and exciting era of further advancements in this field of neuroscience.
Keywords: Parkinson's disease (PD); atypical Parkinsonian syndromes; biomarkers; diffusion-weighted imaging (DWI); magnetic resonance imaging (MRI); positron emission tomography (PET); single photon emission computed tomography (SPECT); transcranial sonography (TCS).
Copyright © 2020 Saeed, Lang and Masellis.
Figures
References
-
- Parkinson's Disease Foundation Statistics. Available online at: https://www.parkinson.org/Understanding-Parkinsons/Statistics (accessed April 14, 2020).
Publication types
LinkOut - more resources
Full Text Sources

