OnabotulinumtoxinA Is an Effective Treatment for Chronic Migraine in Patients With Comorbid Fibromyalgia
- PMID: 33178117
- PMCID: PMC7593548
- DOI: 10.3389/fneur.2020.575130
OnabotulinumtoxinA Is an Effective Treatment for Chronic Migraine in Patients With Comorbid Fibromyalgia
Abstract
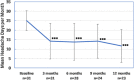
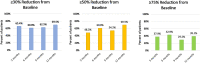
Introduction: Fibromyalgia (FM) is a frequent comorbidity in patients with chronic migraine (CM). PREEMPT trials, which demonstrated the efficacy of OnabotulinumtoxinA (OnabotA) on CM, excluded patients with FM. Our aim was to evaluate the effectiveness of OnabotA in a series of patients with CM and FM. Methods: We analyzed patients with a previous diagnosis of CM and FM who had received sessions of OnabotA quarterly between January 2014 and January 2020 in a specialized Headache Clinic. Primary endpoint was the reduction in moderate to severe headache days at 3, 6, 9, and 12 months. Results: Data were collected from 31 patients with CM and FM that received OnabotA (100% females). Mean age at first procedure was 50.2 ± 11.3 years. Depression (93.5%), other central sensitization syndromes (irritable bowel syndrome, interstitial cystitis, multiple chemical sensitivity, endometriosis, and chronic fatigue syndrome) (48.4%), and medication overuse headache (90.3%) were frequent comorbidities. 48.4% of patients had failed ≥3 preventives previously. The percentage of patients who achieved ≥30 and ≥50% moderate-severe headache reduction on the third month was 65.4 and 48.2%, respectively. Twenty-three patients completed four cycles of treatment, with 13.4 fewer headache days per month than at baseline (p < 0.001). By 1 year, 69.5% had ≥50% reduction of headache frequency and 39.1% had a ≥75% reduction. In 4 cases (21%), OnabotA was interrupted due to a lack of response. Only mild adverse effects were recorded. Conclusion: OnabotA is an effective treatment for CM in patients with FM.
Keywords: central sensitization; chronic migraine; comorbidities; fibromyalgia; onabotulinumtoxinA; quality of life.
Copyright © 2020 Sastre Real and Díaz de Terán.
Figures
Similar articles
-
Real-life data in 115 chronic migraine patients treated with Onabotulinumtoxin A during more than one year.J Headache Pain. 2016 Dec;17(1):112. doi: 10.1186/s10194-016-0702-1. Epub 2016 Dec 12. J Headache Pain. 2016. PMID: 27957623 Free PMC article.
-
Long-term Treatment Benefits and Prolonged Efficacy of OnabotulinumtoxinA in Patients Affected by Chronic Migraine and Medication Overuse Headache over 3 Years of Therapy.Front Neurol. 2017 Nov 3;8:586. doi: 10.3389/fneur.2017.00586. eCollection 2017. Front Neurol. 2017. PMID: 29163347 Free PMC article.
-
OnabotulinumtoxinA treatment for chronic migraine: experience in 52 patients treated with the PREEMPT paradigm.Springerplus. 2015 Apr 13;4:176. doi: 10.1186/s40064-015-0957-z. eCollection 2015. Springerplus. 2015. PMID: 25897415 Free PMC article.
-
[What is the optimal dose for the prophylactic treatment of chronic migraine patients?].Rev Neurol. 2014 Mar 10;58 Suppl 2:S13-9. Rev Neurol. 2014. PMID: 24687880 Review. Spanish.
-
OnabotulinumtoxinA for the prophylactic treatment of headaches in adult patients with chronic migraine: a safety evaluation.Expert Opin Drug Saf. 2021 Nov;20(11):1275-1289. doi: 10.1080/14740338.2021.1948531. Epub 2021 Jul 5. Expert Opin Drug Saf. 2021. PMID: 34187265 Review.
Cited by
-
Risk factors affecting the therapeutic effect of onabotulinum toxin a on chronic migraine.Pain Manag. 2025 Feb;15(2):65-71. doi: 10.1080/17581869.2025.2458448. Epub 2025 Jan 27. Pain Manag. 2025. PMID: 39871598
References
LinkOut - more resources
Full Text Sources