Dynamics of Mono- and Dual-Species Biofilm Formation and Interactions Between Paracoccidioides brasiliensis and Candida albicans
- PMID: 33178146
- PMCID: PMC7591818
- DOI: 10.3389/fmicb.2020.551256
Dynamics of Mono- and Dual-Species Biofilm Formation and Interactions Between Paracoccidioides brasiliensis and Candida albicans
Abstract
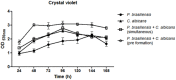
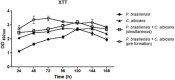
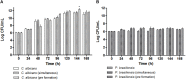
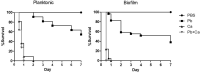
The oral cavity is a highly diverse microbial environment in which microorganisms interact with each other, growing as biofilms on biotic and abiotic surfaces. Understanding the interaction among oral microbiota counterparts is pivotal for clarifying the pathogenesis of oral diseases. Candida spp. is one of the most abundant fungi in the oral mycobiome with the ability to cause severe soft tissue lesions under certain conditions. Paracoccidioides spp., the causative agent of paracoccidioidomycosis, may also colonize the oral cavity leading to soft tissue damage. It was hypothesized that both fungi can interact with each other, increasing the growth of the biofilm and its virulence, which in turn can lead to a more aggressive infectivity. Therefore, this study aimed to evaluate the dynamics of mono- and dual-species biofilm growth of Paracoccidioides brasiliensis and Candida albicans and their infectivity using the Galleria mellonella model. Biomass and fungi metabolic activity were determined by the crystal violet and the tetrazolium salt reduction tests (XTT), respectively, and the colony-forming unit (CFU) was obtained by plating. Biofilm structure was characterized by both scanning electronic- and confocal laser scanning- microscopy techniques. Survival analysis of G. mellonella was evaluated to assess infectivity. Our results showed that dual-species biofilm with P. brasiliensis plus C. albicans presented a higher biomass, higher metabolic activity and CFU than their mono-species biofilms. Furthermore, G. mellonella larvae infected with P. brasiliensis plus C. albicans presented a decrease in the survival rate compared to those infected with P. brasiliensis or C. albicans, mainly in the form of biofilms. Our data indicate that P. brasiliensis and C. albicans co-existence is likely to occur on oral mucosal biofilms, as per in vitro and in vivo analysis. These data further widen the knowledge associated with the dynamics of fungal biofilm growth that can potentially lead to the discovery of new therapeutic strategies for these infections.
Keywords: Candida albicans; Galleria mellonella; Paracoccidioides brasiliensis; dual-species biofilm; oral cavity.
Copyright © 2020 Oliveira, Medina-Alarcón, Singulani, Fregonezi, Pires, Arthur, Fusco-Almeida and Mendes Giannini.
Figures


Similar articles
-
Paracoccidioides spp.: the structural characterization of extracellular matrix, expression of glucan synthesis and associated genes and adhesins during biofilm formation.Front Microbiol. 2024 Mar 7;15:1354140. doi: 10.3389/fmicb.2024.1354140. eCollection 2024. Front Microbiol. 2024. PMID: 38516014 Free PMC article.
-
Relative Abundances of Candida albicans and Candida glabrata in In Vitro Coculture Biofilms Impact Biofilm Structure and Formation.Appl Environ Microbiol. 2018 Apr 2;84(8):e02769-17. doi: 10.1128/AEM.02769-17. Print 2018 Apr 15. Appl Environ Microbiol. 2018. PMID: 29427422 Free PMC article.
-
In vitro Paracoccidioides brasiliensis biofilm and gene expression of adhesins and hydrolytic enzymes.Virulence. 2015;6(6):642-51. doi: 10.1080/21505594.2015.1031437. Virulence. 2015. PMID: 26055497 Free PMC article.
-
Highlights in pathogenic fungal biofilms.Rev Iberoam Micol. 2014 Jan-Mar;31(1):22-9. doi: 10.1016/j.riam.2013.09.014. Epub 2013 Nov 16. Rev Iberoam Micol. 2014. PMID: 24252828 Review.
-
Living together: The role of Candida albicans in the formation of polymicrobial biofilms in the oral cavity.Yeast. 2023 Aug;40(8):303-317. doi: 10.1002/yea.3855. Epub 2023 May 16. Yeast. 2023. PMID: 37190878 Review.
Cited by
-
2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms.Front Cell Infect Microbiol. 2021 May 13;11:679470. doi: 10.3389/fcimb.2021.679470. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34055673 Free PMC article.
-
Paracoccidioides spp.: the structural characterization of extracellular matrix, expression of glucan synthesis and associated genes and adhesins during biofilm formation.Front Microbiol. 2024 Mar 7;15:1354140. doi: 10.3389/fmicb.2024.1354140. eCollection 2024. Front Microbiol. 2024. PMID: 38516014 Free PMC article.
-
Effect of platelet-rich plasma and platelet-rich fibrin on healing of burn wound with dual-species biofilm.Kaohsiung J Med Sci. 2025 Mar;41(3):e12940. doi: 10.1002/kjm2.12940. Epub 2025 Jan 19. Kaohsiung J Med Sci. 2025. PMID: 39829200 Free PMC article.
-
Characterization of Biofilm Formation by the Dermatophyte Nannizzia gypsea.J Fungi (Basel). 2025 Jun 14;11(6):455. doi: 10.3390/jof11060455. J Fungi (Basel). 2025. PMID: 40558967 Free PMC article.
-
Mixed Fungal Biofilms: From Mycobiota to Devices, a New Challenge on Clinical Practice.Microorganisms. 2022 Aug 26;10(9):1721. doi: 10.3390/microorganisms10091721. Microorganisms. 2022. PMID: 36144323 Free PMC article. Review.
References
-
- Baena-Monroy T., Moreno-Maldonado V., Franco-Martinez F., Aldape-Barrios B., Quindos G., Sanchez-Vargas L. O. (2005). Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med. Oral Patol. Oral Cir. Bucal. 10(Suppl. 1), E27–E39. - PubMed
-
- Barbosa M. S., Báo S. N., Andreotti P. F., Faria F. P., Felipe M. S. S., Feitosa L. S., et al. (2006). Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 74 382–389. 10.1128/IAI.74.1.382-389.2006. - DOI - PMC - PubMed
-
- Bartnicka D., Karkowska-Kuleta J., Zawrotniak M., Satala D., Michalik K., Zielinska G., et al. (2019). Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci. Rep. 9:4376. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

