Molecular Epidemiology of Acinetobacter calcoaceticus- Acinetobacter baumannii Complex Isolated From Children at the Hospital Infantil de México Federico Gómez
- PMID: 33178158
- PMCID: PMC7593844
- DOI: 10.3389/fmicb.2020.576673
Molecular Epidemiology of Acinetobacter calcoaceticus- Acinetobacter baumannii Complex Isolated From Children at the Hospital Infantil de México Federico Gómez
Abstract
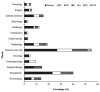
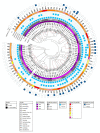
The Acinetobacter calcoaceticus-baumannii (Acb) complex is regarded as a group of phenotypically indistinguishable opportunistic pathogens responsible for mainly causing hospital-acquired pneumonia and bacteremia. The aim of this study was to determine the frequency of isolation of the species that constitute the Acb complex, as well as their susceptibility to antibiotics, and their distribution at the Hospital Infantil de Mexico Federico Gomez (HIMFG). A total of 88 strains previously identified by Vitek 2®, 40 as Acinetobacter baumannii and 48 as Acb complex were isolated from 52 children from 07, January 2015 to 28, September 2017. A. baumannii accounted for 89.77% (79/88) of the strains; Acinetobacter pittii, 6.82% (6/88); and Acinetobacter nosocomialis, 3.40% (3/88). Most strains were recovered mainly from patients in the intensive care unit (ICU) and emergency wards. Blood cultures (BC) provided 44.32% (39/88) of strains. The 13.63% (12/88) of strains were associated with primary bacteremia, 3.4% (3/88) with secondary bacteremia, and 2.3% (2/88) with pneumonia. In addition, 44.32% (39/88) were multidrug-resistant (MDR) strains and, 11.36% (10/88) were extensively drug-resistant (XDR). All strains amplified the bla OXA-51 gene; 51.13% (45/88), the bla OXA-23 gene; 4.54% (4/88), the bla OXA-24 gene; and 2.27% (2/88), the bla OXA-58 gene. Plasmid profiles showed that the strains had 1-6 plasmids. The strains were distributed in 52 pulsotypes, and 24 showed identical restriction patterns, with a correlation coefficient of 1.0. Notably, some strains with the same pulsotype were isolated from different patients, wards, or years, suggesting the persistence of more than one clone. Twenty-seven sequence types (STs) were determined for the strains based on a Pasteur multilocus sequence typing (MLST) scheme using massive sequencing; the most prevalent was ST 156 (27.27%, 24/88). The Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas I-Fb system provided amplification in A. baumannii and A. pittii strains (22.73%, 20/88). This study identified an increased number of MDR strains and the relationship among strains through molecular typing. The data suggest that more than one strain could be causing an infection in some patient. The implementation of molecular epidemiology allowed the characterization of a set of strains and identification of different attributes associated with its distribution in a specific environment.
Keywords: Acinetobacter baumannii; Acinetobacter calcoaceticus-Acinetobacter baumannii complex; intensive care unit; molecular typing; resistance.
Copyright © 2020 Mancilla-Rojano, Ochoa, Reyes-Grajeda, Flores, Medina-Contreras, Espinosa-Mazariego, Parra-Ortega, Rosa-Zamboni, Castellanos-Cruz, Arellano-Galindo, Cevallos, Hernández-Castro, Xicohtencatl-Cortes and Cruz-Córdova.
Figures

References
-
- Alcántar-Curiel M. D., García-Torres L. F., González-Chávez M. I., Morfín-Otero R., Gayosso-Vázquez C., Jarillo-Quijada M. D., et al. . (2014). Molecular mechanisms associated with nosocomial carbapenem-resistant Acinetobacter baumannii in Mexico. Arch. Med. Res. 45, 553–560. 10.1016/j.arcmed.2014.10.006, PMID: - DOI - PubMed
-
- Alcántar-Curiel M. D., Rosales-Reyes R., Jarillo-Quijada M. D., Gayosso-Vázquez C., Fernández-Vázquez J. L., Toledano-Tableros J. E., et al. . (2019). Carbapenem-resistant Acinetobacter baumannii in three tertiary care hospitals in Mexico: virulence profiles, innate immune response and clonal dissemination. Front. Microbiol. 10:2116. 10.3389/fmicb.2019.02116, PMID: - DOI - PMC - PubMed
-
- Andrews S. (2010). FastQC: una herramienta de control de calidad para datos de secuencia de alto rendimiento. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (Accessed April 2, 2020).
LinkOut - more resources
Full Text Sources

