Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields
- PMID: 33178164
- PMCID: PMC7596191
- DOI: 10.3389/fmicb.2020.582779
Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields
Abstract
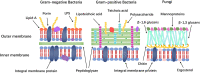
Antimicrobial peptides (AMPs) are a class of small peptides that widely exist in nature and they are an important part of the innate immune system of different organisms. AMPs have a wide range of inhibitory effects against bacteria, fungi, parasites and viruses. The emergence of antibiotic-resistant microorganisms and the increasing of concerns about the use of antibiotics resulted in the development of AMPs, which have a good application prospect in medicine, food, animal husbandry, agriculture and aquaculture. This review introduces the progress of research on AMPs comprehensively and systematically, including their classification, mechanism of action, design methods, environmental factors affecting their activity, application status, prospects in various fields and problems to be solved. The research progress on antivirus peptides, especially anti-coronavirus (COVID-19) peptides, has been introduced given the COVID-19 pandemic worldwide in 2020.
Keywords: antimicrobial peptides; application; classification; coronavirus; design; mode of action; motifs.
Copyright © 2020 Huan, Kong, Mou and Yi.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

